How Safe Are GC and HPLC Columns and Tubing at High Pressures? And When Will They Burst
Norbert Reuter, Technical Manager, Global Technical Support for CSD, Middelburg, The Netherlands
Introduction
Seeing the trends in gas and liquid chromatography- one can conclude that the chromatographer's salvation is based on pressure—the more, the better.
In gas chromatography, we use ever-decreasing internal diameters and, therefore, higher pressure in the carrier gas system to achieve optimum flows/linear velocities. Pressures up to 10 to 15 bar (145 to 220 psi) are normal for 0.10 mm ID columns. For supply gas lines that can also be a lot higher. Where gas supply tubing is made of steel, columns are mostly made of fused silica.
In liquid chromatography, with the appearance of the ultra high-performance LC technology, the working pressure can go up to 1300 bar (18800 psi) or more.
It seems that "How safe are my (GC and HPLC) columns and tubing at high pressures—when do they burst?" is a valid question.
Tough Materials
Comparing sheet metal with glass plates or plastic plates and our household experiences, shows that there is a difference in the toughness of materials. Glass breaks easily, we stretch plastic foil until it ruptures, but steel or other metal foil are strong.
The physical property related to this toughness is the (ultimate) tensile strength. The Wikipedia definition is: "The maximum stress a material can withstand when subjected to tension, compression or shearing. It is the maximum stress on the stress-strain curve". From our observations, the tensile strength for steel must be greater than the one for plastics or glass.
| Material | Ultimate Tensile Strength | |
|---|---|---|
| [MPa] | [psi] | |
| PTFE | 17.5 | 2540 |
| Fused Silica | 48.3 | 7005 |
| Glass | 50.0 | 7250 |
| PEEK | 90.0 | 13050 |
| Nickel | 140 | 20300 |
| Copper | 210 | 30250 |
| Stainless Steel 316 | 534 | 77450 |
Table 1: Ultimate Tensile Strength for some materials
Another factor influencing the toughness is how thick the material is—a thin glass plate breaks easily, but a 6 mm thick plate needs quite a pressure to break. In our chromatographic examples this translates to the wall thickness of the tubing—the thinner the wall, the easier it bursts.
Estimation of the Maximum Allowable Working Pressure for Tubing
There are two possible pressures. The maximum allowable working pressure (MAWP), which is a safe pressure to work with, without the possibility of ruptures or bursting. And there is the burst pressure; the name stands for itself. Normally a factor 4 (four) is assumed between the two. If you quadruple the maximum allowable working pressure, you get the burst pressure for temperatures of the materials' chromatographic application areas. Estimations are based on Barlow's formula:
| PMAWP = (OD - ID)/OD · σUTS/SF = 2 dW/OD · σUTS/SF | ID = Internal Diameter of the Pipe OD = Outside Diameter of the Pipe dW = Wall Thickness of the Pipe σUTS = Ultimate Tensile Strength SF = Safety Factor (1 for burst pressure, 4 for MAWP) |
|
The factor (OD - ID)/OD can also be expressed as 1 - ID/OD, this is 1 minus the ratio between internal and outside diameter of the tubing. |
|
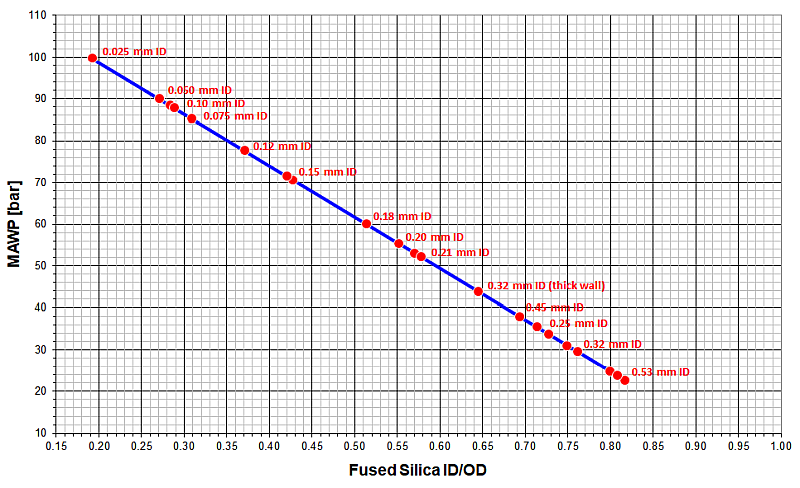
Maximum Allowable Working Pressure (MAWP) for Fused Silica Tubing (without Polyimide layer, safety factor = 4)
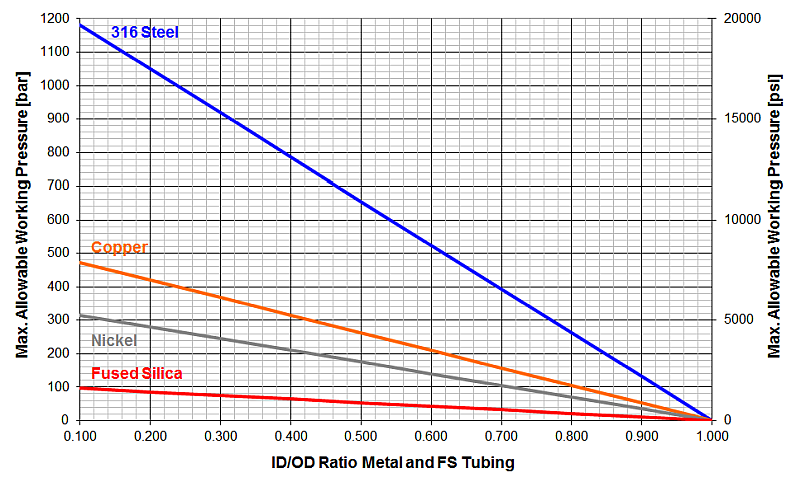
Maximum Allowable Working Pressure (MAWP) for Metal Tubings (FS as Reference), safety factor = 4
| Outer Diameter | Internal Diameter | ID/OD Ratio | |
| [mm] | [inch] | [mm] | |
| 0.80 | 1/32 | 0.50 | 0.625 |
| 1.60 | 1/16 | 0.15 | 0.094 |
| 1.60 | 1/16 | 0.25 | 0.156 |
| 1.60 | 1/16 | 0.50 | 0.313 |
| 1.60 | 1/16 | 0.75 | 0.469 |
| 1.60 | 1/16 | 1.00 | 0.625 |
| 3.18 | 1/8 | 2.00 | 0.629 |
| 3.18 | 1/8 | 2.10 | 0.660 |
| 6.35 | 1/4 | 4.30 | 0.677 |
| 6.35 | 1/4 | 4.40 | 0.693 |
Table 2: Typical dimensions of metal columns
Conclusions
These calculations show why column manufacturers offer their ultra-performance LC columns in special high-pressure hardware with thick steel walls around the packing.
In Gas Chromatography, even a 0.53 mm ID fused silica tubing is safe to use up to 30 bar (430 psig), but it will burst at 120 bar (1750 psig). These pressures can only be achieved with no pressure regulator between gas cylinder and column, so GC columns are safe to use.