In the previous two blog posts (Choosing the right pore size for SEC and Importance of silica particle strength for Sub-2 µm SEC columns), we discussed how the physical properties of the silica, such as pore size, pore volume, and particle strength, affect SEC performance. In this post, we would like to discuss the effect of bio-inertness of bonding chemistry on SEC performance.
The effect of silica particle properties usually seen in chromatograms is related to peak width and resolution. The effect of phase chemistry on the chromatogram is related to peak shape, protein adsorption, and retention time shift of small molecules. One good indication of whether the surface is bio-inert or not is to compare the retention time of uridine, a small neutral molecule, to that of vitamin B12, a small biomolecule. If the B12 retention time is longer than that of uridine, it usually indicates some surface silanol interaction.
For SEC, the characteristics of bonding phase should exhibit:
- Very hydrophilic – No hydrophobic adsorption of proteins nor nonspecific binding
- Full surface coverage – No surface silanol activity, no ion exchange interaction
- Stable phase – Long chemical lifetime, no retention time shift over time
For AdvanceBio SEC 200 Å 1.9 µm, we did bonding optimization to achieve the above goals:
- It is not diol phase. Many SEC column manufacturers use a diol phase. We have found that diol phase is not ideal for ADCs, as the peaks tail. We developed a new hydrophilic bonding chemistry using another hydrophilic group for our 2.7 µm AdvanceBio SEC column, and found it to give better peak shape. 1.9 µm AdvanceBio SEC is based on similar phase chemistry.
- The surface coverage is further optimized for 1.9 µm AdvanceBio SEC. Total coverage of the surface is good, but too much coverage will result in greater loss of pore volume. We optimized the bonding process to balance surface coverage and pore volume reduction after bonding so that we still have inert surface but maintain high pore volume after bonding.
- The phase is crosslinked to ensure chemical stability.
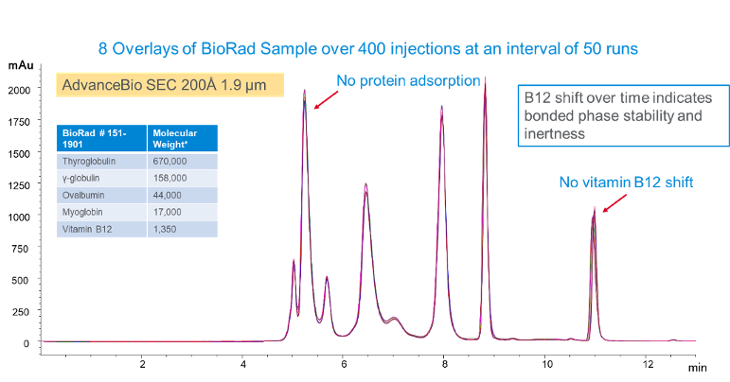
The figure below shows overlays of chromatograms over a lifetime test of 400 injections. There is no protein adsorption and no vitamin B12 shift over 400 injections. If the phase is unstable and changes during the test, B12 will usually start to shift to the right.
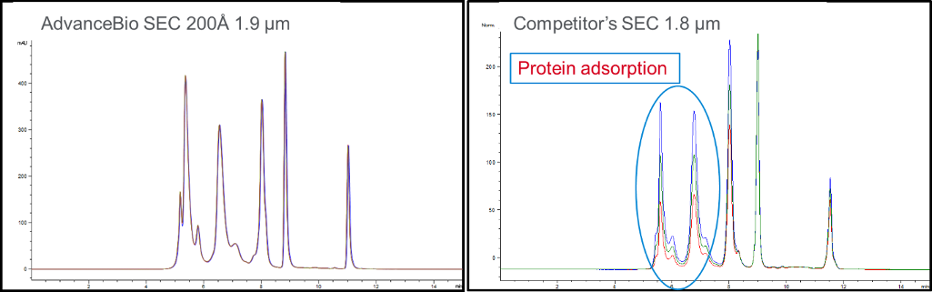
If the phase is too hydrophobic and has surface silanol activity, the large proteins will be adsorbed to surface, and the column has to be so-called “primed”, where the few first injections have to be made to saturate the surface activity before the column can be used. The figure below shows the first five injections of BioRad sample on a fresh column of AdvanceBio SEC 200 Å 1.9 µm and other competitor’s SEC 1.8 µm. There is no need for “priming” the AdvanceBio SEC 200 Å 1.9 µm column, while the competitor’s SEC 1.8 µm column had protein adsorption for the first couple injections and needed priming.
In summary, for BioSEC, bonding chemistry should provide:
- Hydrophilic and bio-inert surface for good peak shape and no nonspecific binding
- Stable phase for column lifetime
---
Thanks, Wu! I always feel more confident about evaluating and troubleshooting my chromatography when I have a better understanding of the “why” behind how my column works. Hopefully this series of posts from Wu help you to make more informed SEC column and method decisions, too.
Until next time -
Anne
Keywords: mAb, monoclonal antibody, ADC, antibody drug conjugates, SEC, size exclusion chromatography, biologics, biosimilars, insulin, molecular weight, aggregation, fragment, quality control, biopharmaceutical, CQA, critical quality attributes, pore size, AdvanceBio, column lifetime, silica, biomolecule, secondary interactions, non specific binding