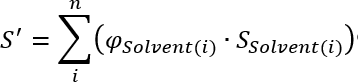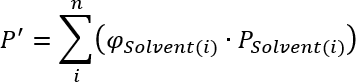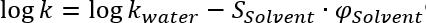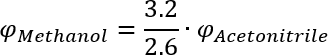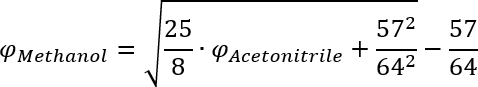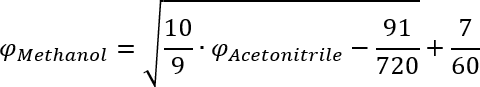Pure solvents as well as solvent mixtures of certain polarities or solvent strengths, i. e. the ability to drag components with it. For the calculation of the polarity of different mixtures Snyder and Kirkland [1] show the following equation:
Solvent Strength Polarity, P Retention
Where the polarity of the mixure (P') is the sum of all individual solvent polarities times the volume fraction of the solvent (φ) . The values for the individual polarities can be found in the following table:
Solvent Solvent Strength, S
for RP PhasesSnyder Polarity, P Acetonitrile 3.2 ±20 % 5.8 Methanol 2.6 ±20 % 5.1 Ethanol 3.6 ±20 % 4.3 Tetrahydrofuran 4.5 ±20 % 4.0 Water 0.0 10.2
For binary mixtures like Acetonitrile/Water or Methanol water the second volume fraction equals (1 - first volume fraction), because the sum of all volume fractions must be 1.
If you want to replace a given Acetonitrile/Water mixture by a Methanol/Water mixture of the same polarity, you have to apply the equation above for both mixtures and set them equal:
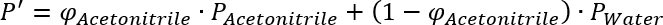 and
and
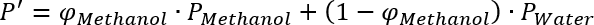
This equation can then be solved for the volume fraction of Methanol and gives the result in the first row of the following table.
for equal polar mixtures
That means you achieve the same polarity if the percentage Acetonitrile is multiplied by 44/51 to get the percentage Methanol (and water) for the given mixture.
The same equations can be used for solvent strength calculations, but because the solvent strength for water is zero, the second term is always zero and the solvent strength depends on the ratio between the solvent strengths of the 2 solvents to be compared (the solvent strength parameter, S, is component dependent.):
Equation Reference Result for
50 % AcetonitrileComment 1 61.5 % simple 2 64.4 % generic 3 77.2 % better performing
for ionizable componentsfor mixtures with equal solvent strengths
Keep in mind that this calculation will result in analyses with the same analysis time (run time), but that retention factors can vary, due to different interactions of the organic modifier with the sample component.
For isoeluotropic mixtures (same retention) the third equation from above can be used, log(kwater) is the retention in pure water and therefore a constant for the same component.
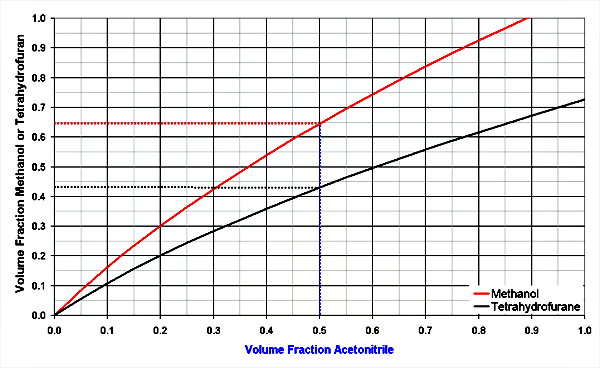
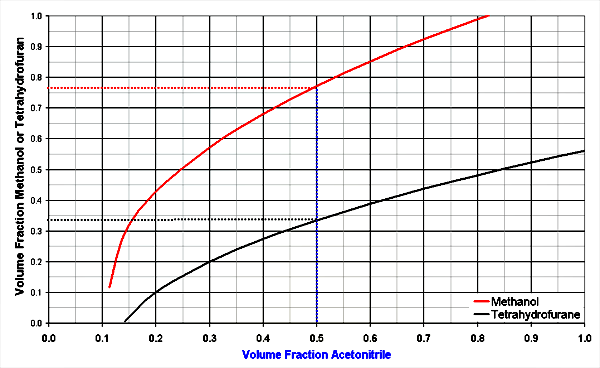
The diagrams below shows 3 solvent mixtures of organic solvents with water. The organic solvents are methanol, acetonitrile and tetrahydrofuran.
Example 1: Which solvent mixtures have similar strength, resulting in the same retention factors?
All components [2]:
Look at 50 % acetonitrile in water (x-axis), the methanol line crossed at 64 % methanol (x-axis) for the same solvent strength. THF is stronger, it only needs 42 % THF in water for the same strength.
Better for ionizable components [3]:
Look at 50 % acetonitrile in water (x-axis), the methanol line is crossed at 76 % for the same solvent strength. THF is stronger, it only needs 34 % THF in water for the same strength.
Example 2: What happens with the retention when changing the percentage organic modifier?
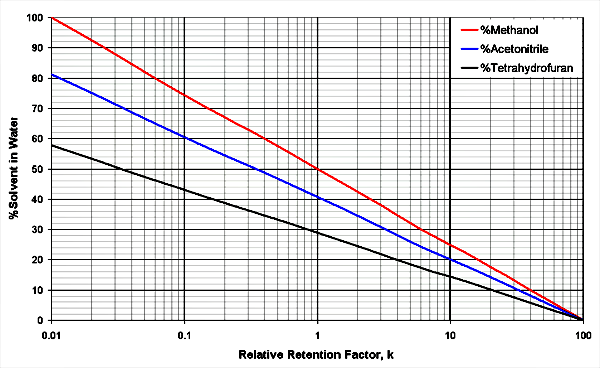
In the diagram above relative retention factors are used, these are numbers to multiply to real retention factors when changing the organic modifier concentration. The 40:60 mixture acetonitrile/water is set as 1.0. Changing the acetonitrile concentration to 50:50 (stronger solvent mixture), changes the retention factor from 1.0 to 0.28 - all peak elute earlier. If the mixture is a 20:80 acetonitrile/water mix, the retention is 10-fold - all peak elute later.
SELECTIVITY
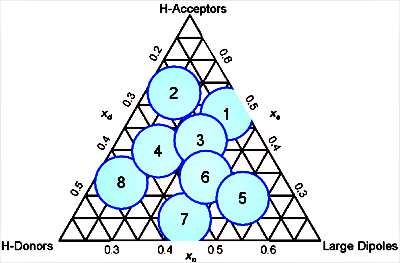
Different solvents show different selectivity's towards some components. Snyder presented his selectivity triangle in [1] showing the 8 classes of solvents:
Group Chemical Group 1 aliphatic ethers, trialkylamines 2 aliphatic alcohols 3 pyridin derivates, THF, amides, glycol ethers, sulfoxides 4 glycols, benzylalcohol, acetic acid, formanide 5 dichloromethane, ethylene chloride 6 aliphatic ketones and esters, dioxane, nitriles, aniline 7 aromatic hydrocarbons, halogenated aromatics, nitro-compounds, aromatic ethers 8 fluoroalcohols, water, chloroform Each class is a combination of 3 interactions in various percentages connected to hydrogen donors, hydrogen acceptors and large dipoles. Water is part of group 8, acetonitrile is group 6, methanol is group 2 and THF is group 3.
If acetonitrile is replaced by methanol (or THF), there may be a different elution order for the components, maybe not within a chemical class (like polycyclic aromatic hydrocarbons), but in mixtures with components bearing different functional groups, like in for example pesticide analyses.
Only solvents within the same group should show no selectivity change. If acetonitrile is replaced by propanenitrile (propionitrile) everything should look the same, unfortunately propanenitrile is 500 times more toxic than acetonitrile and therefore its use is limited.
EXAMPLE
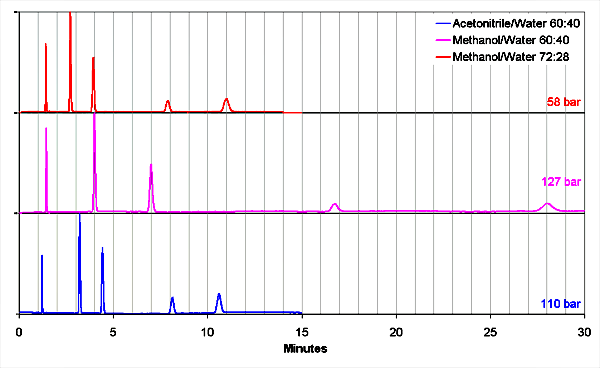
The figure shows that replacing acetonitrile with methanol without change of the percentage (purple line) results in 2.5 times longer retention times and the working pressure doubles. After the methanol percentage is adjusted (blue line), retention times are equal, but selectivities change. Peaks 2 and 3 show more retention, peak 5 less. The components used are uracil, acetophenone, methylbenzoate, toluene and naphthalene.
REFERENCES
- L. R. Snyder, J. J. Kirkland; "Introduction to Modern Liquid Chromatography", Wiley-Interscience, New York 1979.
- P. J. Schoenmakers, H. A. H. Billiet, L. de Galan; J. Chromatogr. 205 (1981) 13.
- P. R. Haddard, S. Sekulic, C. J. Lamberton; J. Chromatogr. 363 (1986) 125.