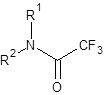
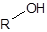Part 1 – Gas Chromatography
N. Reuter*, ACG Global Technical Support for CSD, Middelburg, The Netherlands
Introduction
Wikipedia defines “derivatization“ as follows:
Derivatization is a technique used in chemistry, which transforms a chemical compound into a product of similar chemical structure, called a derivative.
Generally, a specific functional group of the compound participates in the derivatization reaction and transforms the educt to a derivative of deviating reactivity, solubility, boiling point, melting point, aggregate state, or chemical composition. Resulting new chemical properties can be used for quantification or separation of the educt.
Derivatization techniques are frequently employed in chemical analysis of mixtures
That means derivatization is “wet“ chemistry. Though chromatographers may not prefer this type of chemistry, it is quite often used for specific purposes, as mentioned in the first paragraph of the word definition. Something similar, but different.
Alkylation/Esterification Reactions
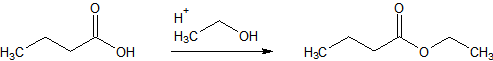
I can still remember a chemical reaction from my basic chemistry classes transforming horrible smelling butanoic acid into an ester with pineapple-like odor. So our ugly educt was butanoic acid, the transforming agent was ethanol (and a catalyst) and the beautiful product was butanoic acid ethylester or ethyl butanoate.
Figure 1: Esterification of butanoic acid.
Let’s look at the physical properties of these two components.
| Butanoic Acid | Ethyl Butanoate |
|---|---|---|
Melting Point | -8 °C | -93 °C |
Boiling Point | 164 °C | 121 °C |
Hydrophobicity, Log(P) | 1.66 | 0.77 |
- There is a decrease in boiling point of more than 40 °C and a decrease in melting point by more than 80 °C
- The quite polar acid is transformed to an apolar and volatile ester
In gas chromatography we have to evaporate our components, so a low boiling point and/or a high volatility/vapor pressure is what we want and – of course – thermal stability.Esterifications can be used on all types of organic or even inorganic acids to get a volatile product. An example for an inorganic acid is boric acid (H3BO3) with a melting point of 169 °C under decomposition and therefore no boiling point. Derivatization with a methylation agent to trimethyl borate gives a melting point of -34 °C and a boiling point of 69 °C, and therefore the possibility to analyze it with GC (plus there is no more thermal decomposition).The most often found methylation today is the transformation of fat/fatty acids into fatty acid methyl esters (FAME) or biodiesel (a transesterification reaction from a glycerol ester to methyl esters).The classical derivatization agents used were diazomethane or borontrifluoride/methanol, the first a highly explosive reagent, the latter a quite aggressive one. Today the reaction with trimethylsulfonium hydroxide (TMSH) is preferred. This is due to the simplicity of mixing sample with reagent and injecting it directly into the heated injector, where the reaction immediately takes place. For a comparison see the following two procedures for the derivatization of fatty acids or triglycerides to FAME’s:
| Reagent | Procedure |
|---|---|
| Diazomethane | Use a diazomethane generator for 1 μmol diazomethane or obtain diazomethane from the decomposition of N-Nitroso-N-methylurea, N-Nitroso-N-methyl-p-toluenesulfonamide or N-methyl-N'-nitro-N-nitrosoguanidine (Diazald), then add the ethereal diazomethane solution to an ethereal solution of the sample until a permanent yellow color occurs. For fatty acids 30 minutes at 0 °C. |
| BF3/Methanol | To 100 mg of the organic acid in a 5-mL vial add 3 mL BF3/methanol. Heat to 60 °C for 5-10 minutes. Cool and transfer to a separatory funnel with 25 mL hexane. Wash twice with saturated sodium chloride solution, dry with anhydrous sodium sulfate and evaporate solvent. Inject. |
| TMSH | Typically the methylation of triglycerides is performed with a 1% solution of lipids together with half the volume of a 0.2 molar TMSH solution. The reaction mixture can be directly analyzed with GC after a short heating period at 100 °C or inject directly into a GC injector at a minimum of 250 °C. |
Silylation Reactions
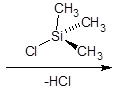
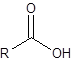
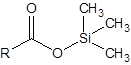
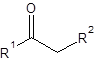
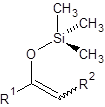
The silylation is the most widely used derivatization technique in gas chromatography. All types of functional groups can be derivatized by these reagents, as long as there is an acidic hydrogen somewhere in the molecule. Alcohols and phenols, thiols, amines, organic acids and CH-acidic components can be derivatized.There are lots of different reagents like MSTFA, BSTFA, TMS, TMSI, TMSDEA, MSHFBA, HMDS, DERIVA-SIL and TRI-SIL. They all have in common that the transferred group is trimethylsilyl (TMS, -SiMe3)
Educt | TMS-Cl | Product |
Alcohol |
| Silyl ether |
Amine | Silyl amine | |
Acid | Silyl ester | |
CH-acidic Carbonyl compound | Silyl enol ether |
A typical procedure for Bis-(trimethylsilyl)-trifluoroacetamide (BSTFA) is: To 5-10 mg of sample in a vial add 0.5 mL silylation reagent followed by 1 mL of derivatization grade solvent. (Sometimes additional solvent is necessary). Derivatization occurs normally with solution or upon warming to 60 °C for 10-15 minutes. Amino acids may require reaction in a sealed tube or vial and heating near the boiling point of the mixture until a clear solution is obtained.
Silyl groups add a lot of volatility into the educt molecule despite the increase in molecular weight. Sugars are a good example. If you heat an underivatized sugar, you will get either a caramel-like product or charcoal, but no evaporation. After trimethylsilylation of all hydroxyl groups in the sugar, they are volatile enough to be analyzed by GC with evaporative injection without decomposition.
Acylation Reactions
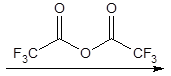
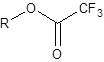
All components bearing an active hydrogen like alcohols, primary and secondary amines, and thiols can be converted into the corresponding ester, amide or thioester by the reaction with an acid anhydride or acid chloride. Most widely used are the anhydrides of acetic acid and perfluoroacetic, -propanoic and -butanoic acid and fluoroacylimidazoles, which are the imidazole amides of the above mentioned fluorinated acids. Their biggest advantage is that after the reaction there is only imidazole remaining in the reaction vessel, which is quite inert compared to the free perfluorinated acid, which is formed after the reaction with an anhydride.AA = acetic acid anhydride, TFAA = trifluoroacetic acid anhydride; PFPA = pentafluoropropanoic acid anhydride and HFBA = heptafluorobutanoic acid anhydride. The fluorinated anhydrides are abbreviated as TFA-derivatives, PFP-derivatives and HFB-derivatives.
The perfluorinated anhydride offers one more advantage for GC. They introduce a trifluoromethyl group into the molecule, which gets visibility for an Electron-Capture Detector (ECD) – a detector that is selective for molecules with strongly electronegative functional groups like halogens or nitro groups.Another advantage is a fragmentation-directing effect in mass spectrometric detections of proteomics and other bioorganic samples.
A typical procedure for trifluoroacetylation is: Dissolve 50 μg (ECD) or 250 μg (FID) of sample in 0.5 mL derivatization grade benzene in a 5-mL vial. Add 0.1 mL of the 0.05M-trimethylamine/benzene solution followed by 10 μg of the anhydride. Cap the vial and heat for 15 minutes to 50 °C. Cool and add 1 mL of a 5 % aqueous ammonia solution. Shake for 5 minutes and separate benzene layer. Inject directly.
Combination Methods
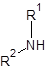
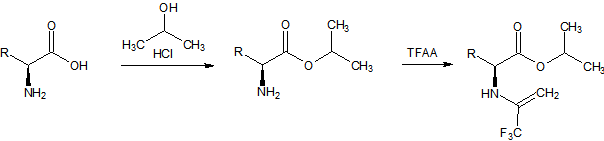
Sometimes multiple derivatizations have to be carried out, due to the structure of the target molecules. A good example is the class of amino acids. Carboxylic acids that also carry a basic amino group and that are – at least in aqueous solutions – zwitter-ionic.The derivatization of amino acids is a two-step process. First the carboxyl group is converted to an ester and second the amino group is converted into an amide:

Figure 2: Two-step derivatization of amino acids. The reaction products are thermally stable and volatile and can be injected to be analyzed for example on chiral columns, like CP-Chirasil-L-Val for enantiomeric purity of the corresponding amino acid.
The typical procedure is: First step esterification: A 1 mL volume of 2M-HCl in 2-propanol is added to a dry sample. Air is displaced with nitrogen. 1 mL of ethanethiol is added to prevent oxidative decomposition of trytophan and to convert cystine into cysteine. The reaction mixture is heated at 110 °C for an hour. Solvent and excess reagents are removed with a gentle stream of nitrogen.Second step acylation: A 250 μL volume of acetic acid ethylester and 50 μL of TFAA are added and the reaction mixture is heated at 110 °C for 10 minutes. If arginine is to be determined, the temperature must be increased to 150 °C. Solvent and excess reagents are evaporated with a gentle stream of nitrogen. The residue is dissolved in a known volume of dichloromethane and can be injected directly.
This also shows that derivatization procedures can be quite complex.
Drawbacks
If you inject a sample together with unreacted derivatization reagent or their reaction product, it can happen that the column bleed increases quite a bit. Siloxane phases always carry free silanol groups and these act like alcohol groups and can be derivatized and chains can be broken. Also all trimethylsilylation reagents give a specific reaction product – hexamethylcyclotrisiloxane, D3 – which is also the main component measured as column bleed and it is therefore misidentified as such.
Conclusions
Derivatizations in gas chromatography are used to:
- Decrease boiling points
- Increase volatility/vapor pressures
- Enhance thermal stability
- Enhance response for certain detectors
Derivatization reaction can be as simple as adding the reagent and injecting, or as complex as the two-step approach for amino acids.
References
I. V. Tetko, J. Gasteiger, R. Todeschini, A. Mauri, D. Livingstone, P. Ertl, V. A. Palyulin, E. V. Radchenko, N. S. Zefirov, A. S. Makarenko, V. Y. Tanchuk, V. V. Prokopenko; “Virtual computational chemistry laboratory - design and description”, J. Comput. Aid. Mol. Des., 19, 2005, 453-63.
VCCLAB, Virtual Computational Chemistry Laboratory, http://www.vcclab.org, 2005.
Recommended Literature
D. R. Knapp; “Handbook of Analytical Derivatization Reactions”, Wiley-Intersciece, New York 1979. ISBN 978-0471034698.
J. Drozd; “Chemical Derivatization in Gas Chromatography” (J. Chromatogr. Library Vol. 19), Elsevier Scientific, Amsterdam 1981. ISBN 978-0444419170.
K. Blau, J. M. Halket; “Handbook of Derivatives for Chromatography”, Wiley-Interscience, New York 1993. ISBN 978-0471926993.
T. Toyo'oka; “Modern Derivatization Methods for Separation Science”, Wiley-Interscience, New York 1999. ISBN 978-0471983644.
S. C. Moldoveanu, V. David; “Sample Preparation in Chromatography” (J. Chromatogr. Library Vol. 65), Elsevier Scientific, Amsterdam 2002. ISBN 978-0444503947.
Part 2 of this document is about derivatizations in liquid chromatography.