Introduction
Some pesticides are reported to be thermo-sensitive. As a result, they may show (partial) degradation in the injector. This process is in some cases aggravated by activity in the liner. The list includes:
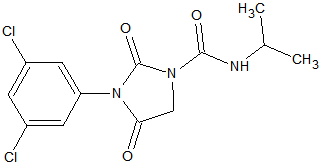
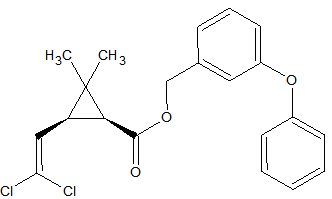
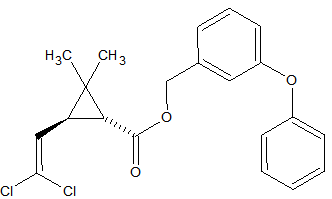
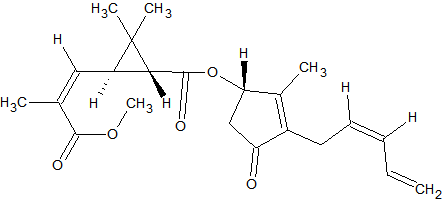
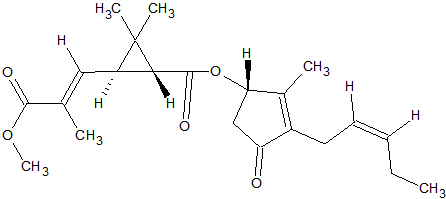
N-methylcarbamate Pesticides
The N-methylcarbamate pesticides can only be analyzed by GC under special conditions. They are thermo-labile and as a consequence will degrade almost completely in a hot split/splitless injector. Degradation occurs also inside the column because of thermal stress. This applies to the following carbamates [9, 14, 15]:
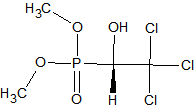
Endrin and DDT
Degradation commonly occurs in active or poorly deactivated injection port liners. 4,4’-DDT degrades into 4,4’-DDE and 4,4’-DDD, Endrin into Endrin ketone and Endrin aldehyde. The EPA protocol SW-846 [6] stipulates the regular check for Endrin and DDT degradation products. If the breakdown exceeds the 15 % level action must be taken. The breakdown is especially a concern for injection methods with an extended sample contact with the liner; these methods include splitless and large volume injections. Various types of commercially available specially deactivated liners can limit the breakdown level.
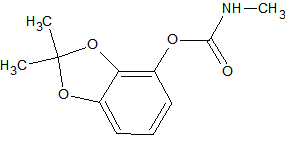
Other Pesticides
Pesticide
Ref.
Structure
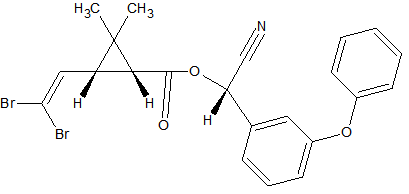
Amitraz
2
sensitive to hydrolyzation during extraction
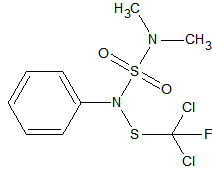
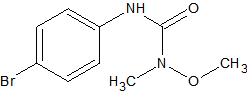
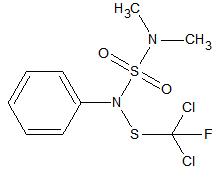
Carbosulfan
2
sensitive to matrix induced decomposition in the liner
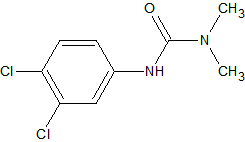
Dichlofluanid
2
may degrade by chemical reactions with thiol groups in the matrix during extraction
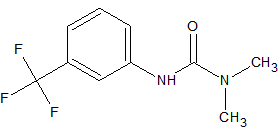
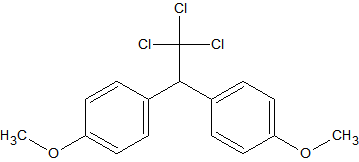
Methoxychlor
also reported as a thermo-labile pesticide
Phoxim
2
sensitive to matrix induced decomposition in the liner
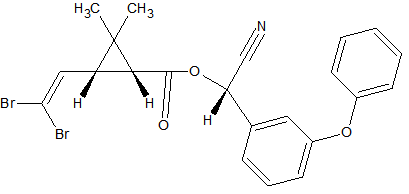
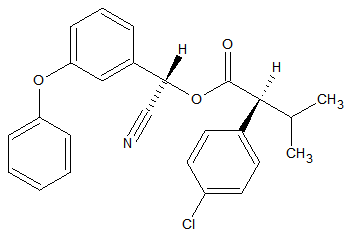
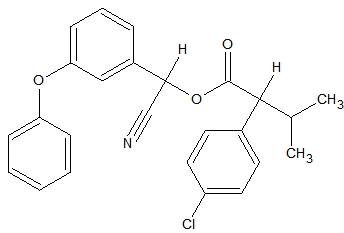
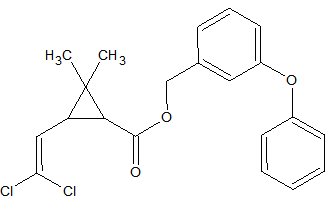
Synthetic pyrethroids
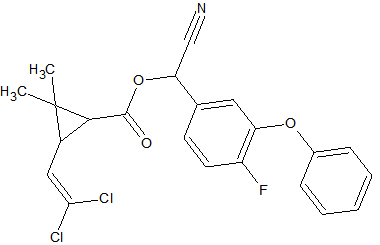
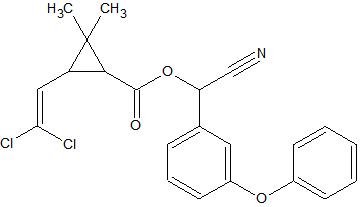
Some synthetic pyrethroids seem to show breakdown induced by the liner. This is caused by poor liner deactivation or by active glass wool. Also an increase in recovery of 50-140% has been observed for Cypermethrin, Deltamethrin and Fenvalerate due to matrix influences. Possibly through liner deactivation by the matrix. Tetramethrin [3] seems less sensitive for degradation. Esfenvalerate, cis-Permethrin and trans-Permethrin have a limited storage time in natural water samples. It is advised to analyze these within 24 hours of sample collection. The list of affected pyrethroids include:
Lambda-Cyhalothrin is converted inside the liner into its cis-A isomer by epimerization. The liner materials, quartz or borosilicate glass, seem of major influence. Lambda-Cyhalothrin is also degraded inside the column at temperature above 240 °C. These results are based on the analyses of pure components without any matrix influence [4].
Natural pyrethroids
The GC analysis of natural pyrethroids is critical. Good analytical results can be obtained using on-column injections, short columns and high column flows thus limiting the thermal stress [8].
References
-
P. Sandra, J. Chromatogr. A 2003, 1000, 299.
-
Validation of Multiresidue Screening Methods for the Determination of 186 Pesticides in 11 Agricultural Products Using Gas Chromatography;
Y. Hirahara et al., J. Health Sci. 2005, 51(5), 617-627. -
Effect of Injector Conditions on the Reproducibility of Gas Chromatographic-Electron Capture Detection of Sterol Derivatives and Synthetic Pyrethroids.
L. Y. Jayasinghe, P. J. Marriott, A. M. Nguyen, J. High Resol. Chromatogr. 1999, 22(6), 362-366. -
Tony Baker, Syngenta, Jealotts Hill International Research Centre, 2006. Personal communication.
-
Evaluation of Automated and Manual Hot-Splitless, Cold Splitless (PTV), and On-Column Injection Technique Using Capillary Gas Chromatography for the analysis of Organophosphurus Pesticides; H.-M. Müller, H.-J. Stan, J. High Resol. Chromatogr. 1988, 11, 140-143.
-
Environmental Test Methods and Guidelines, Environmental Protection Agency
-
Matrix-induced effects: a critical point in the gas chromatographic analysis of pesticide residues; J. Hajšlová, K. Holadova et. al., J. Chromatogr. A 1998, 800, 283-295.
-
Optimized Gas Chromatographic Analysis of Natural Pyrethrins and Pyrethroids; T. J. Class; J. High Resol. Chromatogr. 1991, 14 (1), 48-51.
-
Thermal Degradation Observed with different Injection Techniques: Quantitative Estimation by the Use of Thermo-labile Carbamate Pesticides; H.-M. Müller, H.-J. Stan; J. High Resol. Chromatogr. 1990, 13, 759-763.
-
New Method for the Analysis of Pyrethroid Insecticides: Esfenvalerate, cis-Permethrin and trans-Permethrin in Surface Waters Using Solid-Phase Extraction and Gas Chromatography; M. J. Hengel, C. R. Mower, T. Shibamoto, Bull. Environ. Contam. Toxicol. 1997, 59, 171-178.
-
Optimization ans application of the PTV injector for the analysis of pesticide residues; M. Godula, J. Hajšlová, K. Maštouska, J. Krivánková, J. Sep. Sci. 2001, 24, 355-366.
-
Matrix-Induced Effects in Analysis of Trace Pesticides in Sediments by GC-MS; K. Ito, M. Yamauchi, T. Mukai, K. takimoto, M. Okada, Anal. Sci. 2001, 17 Suppl., i921-i923.
-
Compendium of Pesticide Common Names; A. Wood 2006, http://www.alanwood.net/pesticides/
-
Determination of thermo-labile urea pesticides after derivatization with HFBA using GC-ECD and confirmation by means of GC-MSD; H.-J. Stan, P. Klaffenbach, Fresenius' J. Anal. Chem. 1991, 339(1), 40-45.
-
Pesticide residue analysis in food with CGC - study of long-term stability by the use of different injection techniques; H.-M. Müller, H.-J. Stan; J. High Resol. Chromatogr. 1990, 13, 697-701.