Consistently prepared mobile phases are key to consistent chromatography results. Order of operations can matter to more than just math.
A method is only as robust as each of the components involved, and that includes the mobile phases. Preparing mobile phases may seem really simple, and often it is, but sometimes how you go about it really matters.
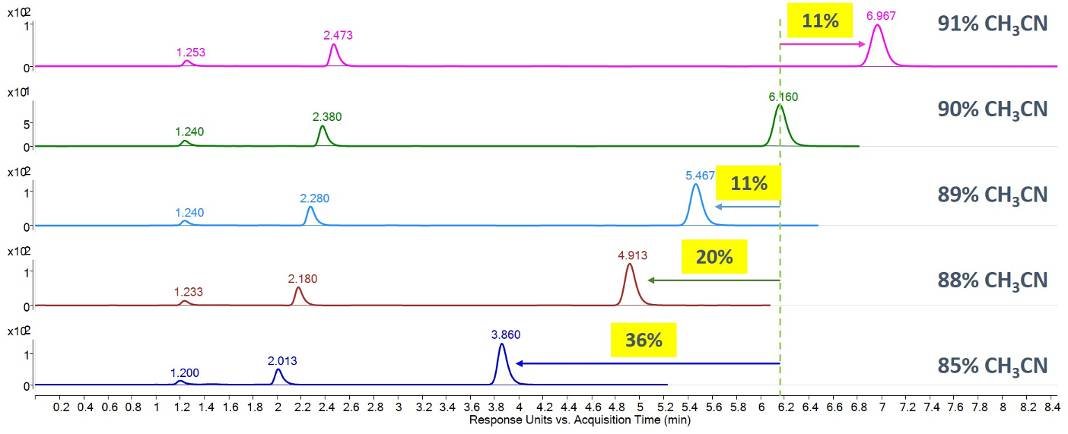
With HILIC in particular, a very small change in the mobile phase composition can have a big impact. Looking at the chromatograms below, you can see that only 1% changes in the mobile phase composition can lead to large changes in retention time.
Isocratic separation of the toluene, cytosine, and uracil mix used for QC on AdvanceBio MS Spent Media columns.
If you look at methods in the user guide or application note for the AdvanceBio MS Spent Media column, a zwitterionic HILIC column, you’ll notice that the mobile phases are often written as follows:
Mobile Phase A = 10% 200 mM ammonium formate in water pH 3, 90% water
Mobile Phase B = 10% 200 mM ammonium formate in water pH 3, 90% acetonitrile
Final salt concentration is 20 mM.
It would be completely reasonable to look at this and think, “Why wouldn’t I just make 20 mM ammonium formate in water in the first place?”
There are a few reasons to make the mobile phases as described above, making a 200 mM stock solution of ammonium formate (or salt you’re using), and then diluting it ten-fold with either water or acetonitrile for mobile phases A and B, respectively.
- Salts typically used for HILIC are easily dissolved in water, but have limited solubility in acetonitrile, so preparing a stock aqueous solution minimizes any solubility challenges.
- Working from a stock solution, and then carefully diluting, ensures that both mobile phases have the same ionic strength. We want to avoid adding an ion exchange effect to our HILIC separation.
- The last reason is simple convenience. It’s faster and easier to make one concentrated salt solution, filter it, and store it in the fridge, than to make two separate mobile phases repeatedly. Filtered and refrigerated salt solutions will often keep for a couple weeks, easily.
Recently I heard from a customer how their lab prepares HILIC mobile phases - they do it gravimetrically - weighing out the solvents in addition to the salts. This is another great approach to very consistent mobile phase preparation.
One last point – while the HILIC mechanism isn’t terribly well understood, it does depend upon analytes partitioning in and out of an aqueous layer on the surface of the stationary phase. It is recommended to always have a minimum of 3% water present to maintain the aqueous layer. Including water in the organic mobile phase (10% in the example above) guarantees that sufficient water is present.
That’s it for this week. We’ll talk more about solvents for HILIC separations next time.
Speak soon -
Anne
Keywords: Bio columns, liquid chromatography, tips and tricks, HILIC, LC mobile phase, AdvanceBio blog