ADCs often post additional challenges beyond those posed by mAbs - learn what to watch out for and how to adjust your method.
Hello again, everybody. This week my colleague Andy is back to talk about HIC method development for ADCs. As if mAbs weren’t tough enough, ADCs present another level of complexity, and require special consideration to be sure that you get accurate results.
--
Analyzing ADCs by HIC
ADCs often post additional challenges beyond mAbs - learn what to watch out for and how to adjust your method
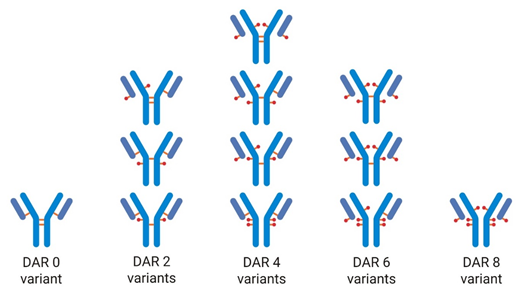
The incorporation of hydrophobic drug molecules into a monoclonal antibody (mAb) forming an antibody drug conjugate (ADC) results in numerous different molecular species with varying degrees of hydrophobicity. This means hydrophobic interaction chromatography (HIC) is an ideal technique for analyzing the all-important drug to antibody ratio (DAR).
However, the range of hydrophobicity presents certain important challenges:
- Sample preparation must result in complete dissolution of the sample.
- HIC gradient conditions must result in complete elution of all variants.
Too much ammonium sulfate in the sample solution could result in DAR 6 and DAR 8 variants not being fully dissolved which may result in an underestimated DAR value. Similarly, the gradient conditions need to ensure elution of all DAR species. This elution is achieved by including propan‑2‑ol as organic modifier.
By choosing the appropriate mobile phase and gradient conditions, it is possible to separate ADC DAR variants in a very short analysis time using new Agilent AdvanceBio HIC columns (Application Note 5994-0149EN has more details).
--
Thanks again to Andy and Sandeep! I’ll be back in two weeks to wrap up our HIC series with some tips and reminders to keep HIC running smoothly.
Keywords: Bio columns, liquid chromatography, hydrophobic interaction chromatography, HIC, method development, ADCs, AdvanceBio blog