Lipid removal is essential for ensuring efficient and accurate results. Discover how Agilent’s Captive EMR-lipid provides a reliable and streamlined option.
Lipids can have a huge impact on LC/MS/MS analysis, becoming the bane of many lab technicians’ lives. The two main issues they cause are: an inability to meet detection criteria and low reproducibility of results.
The presence of lipids can lead to longer method development times, increased data variability, and low data accuracy. Not to mention the time needed to troubleshoot these problems. The challenges are problematic if you’re working with compounds that coelute with lipids.
Lipids can also impact the cleanliness of the mass spectrometer causing detector contamination if they pass through the column. This contamination results in the need to clean the source and to clean or replace the capillary. The instrument must be shut down for cleaning, which leads to downtime of anything between four hours and a day.
A build-up of lipids on the HPLC column impacts the column longevity and can also affect the backpressure. The build-up means that column flushing and re-equilibration is needed, involving a high-organic column wash, causing over two hours of downtime.
Therefore, sample preparation is needed to thoroughly remove the lipid interferences. However, it is typically the most difficult and time-consuming step, and the single biggest time constraint that analytical labs face.
When developing a sample preparation method, there are four key goals:
- Provide high interference removal
- Allow high analyte recovery
- Be effective within the existing workflow
- Be easy for technicians to implement – requiring minimal method development
A valuable solution
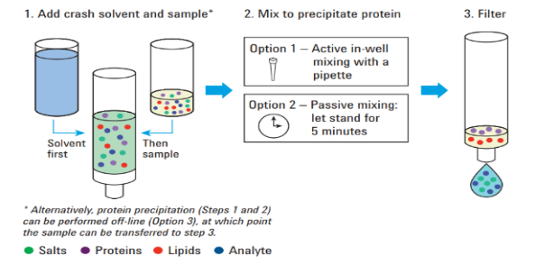
Agilent’s Captiva EMR–Lipid technology can help technicians to achieve effective lipid removal without any analyte loss, improving the quality of their results. Captiva simplifies sample processing by starting protein precipitation in the cartridge. The simple graphic demonstrates the procedure.
The technology is able to produce cleaner samples without the interferences, resulting in better chromatography and lower limits of detection. The innovative sorbent in Captiva EMR–Lipid cartridges and plates captures ion-suppressing lipids, while allowing bulky analyte particles of interest to pass through, as illustrated in the following figure. Agilent’s recent webinar explains the technique and the technology in detail, watch it here.
Who should use Captiva EMR-Lipid cartridges?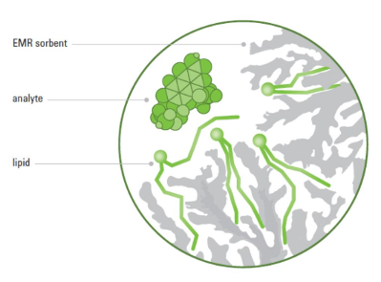
For any research labs working with serum and plasma samples, whether human or animal, the use of Captiva EMR–Lipid technology can be an opportunity to improve workflows. This includes clinical research organizations, coroners, medical examiners, forensic labs, and veterinary research labs.
Lipid removal in action
Lipids from biological samples are detrimental to the research analysis of 25-hydroxyvitamin D2 and D3 metabolites. However, solid phase lipid extraction is time-consuming, involving multiple steps and method development. Whereas typical sample preparation techniques, including protein precipitation, don’t remove lipid interferences. To overcome these issues, a method using Agilent Captiva EMR–Lipid was implemented for in situ protein precipitation and pass-through lipid clean-up.
Precipitated proteins are filtered as the eluent passes through the EMR–Lipid sorbent for clean-up. Unlike commercially available lipid removal products, Captiva EMR–Lipid is highly resistant to clogging. Lipids are removed through a combined mechanism of size-exclusion and hydrophobic interaction, delivering purified eluents ready for analysis.
Using the Captiva EMR-Lipid method, reproducibility was improved significantly. The RSD values were lowered from over 25 % to just 3 % when compared to the same sample with just protein precipitation.
This Application Note describes the full sample processing and experimental process, in which excellent accuracy, precision, and clean-up results were achieved. The method is simple to perform, fast, and provides superior matrix removal ensuring maximum analytical sensitivity, minimal carryover, and high reproducibility.
Generating quality data
These challenges are just some of the issues research technicians working with samples containing lipids can face. Try using Captiva EMR–Lipid and see how much better your analytical data quality is.
For Research Use Only. Not for use in diagnostic procedures
Keywords: Sample prep, data quality, Captiva, lipids, LC-MS/MS, reproducibility, HPLC columns, LC columns, mass spec, liquid chromatography, mass spectrometry, EMR-lipid,