Spoiler - rinse, rinse, rinse! Look at where to pay special attention to mobile phase flow paths.
This week I’m back to wrap up our series of posts on HIC with some tips to keep your HIC method running smoothly. The alternate title for this post would be simply, “Rinse!” As my colleagues Andy and Sandeep have explained, there’s a lot of salt in HIC mobile phases, and the key to avoiding problems with HIC is to be diligent about rinsing and avoiding salt precipitation.
The most important piece of everything I’ll discuss today is this: always be sure to store your column in an appropriate solution! For the Agilent AdvanceBio HIC column, we recommend that you do not store the column in high salt mobile phase. For overnight storage, it is advisable to flush the column with low salt-containing buffer. For longer term storage, you must flush the salt out to store it in 100% acetonitrile. When you switch from high salt buffer to acetonitrile, you’ll need an intermediate flush with water or low concentration buffer to avoid salt precipitation. Also keep an eye on the back pressure, since higher viscosity mobile phases will result in higher pressure. The column user guide describes this is more detail. Every vendor will recommend slightly different storage conditions for their column – so best to always check the user guide.
Next let’s look at how we suggest configuring your mobile phases in the Quick Start Guide for our new HIC column:
Mobile phase A: Low salt mobile phase
Mobile phase B: High salt mobile phase
Mobile phase C: Isopropanol or storage solution Mobile phase
D: Water
First thing you’ll notice is that this is configured for a quaternary pump. If you’re working with a binary pump with two pairs of channels, you’ll want to put water on channel B2 for rinsing. We’ve purposely listed water as mobile phase D on all the guides and application notes for this HIC column to reinforce the importance of rinsing both the column and the LC stack. Never leave the instrument or the column idle under high-salt conditions. Get in the habit of flushing the LC stack with water at the end of your worklist or sequence. Columns may be stored short term under low salt conditions (i.e. Mobile Phase A), but for long-term storage 100% acetonitrile is recommended.
The next habit to form is to regularly check your solvent flowpath for leaks. This is a good habit for all types of chromatography but can be especially key for high salt methods. Even a small leak with 2 M salt can quickly leave the site of the leak crusted over with salt, as can be seen in Figure 1.
Figure 1. A salty mess
Most newer LC models have washing tools integrated into the software and hardware, including a seal wash for the pumps and a needle wash for the autosampler needle. Use them both. At a minimum, this seal wash should be water, ideally water with 10 % isopropanol to prevent microbial growth. Figure 2 shows the mess you could end up with without the needle wash.
Figure 2. A harder-to-clean salty mess
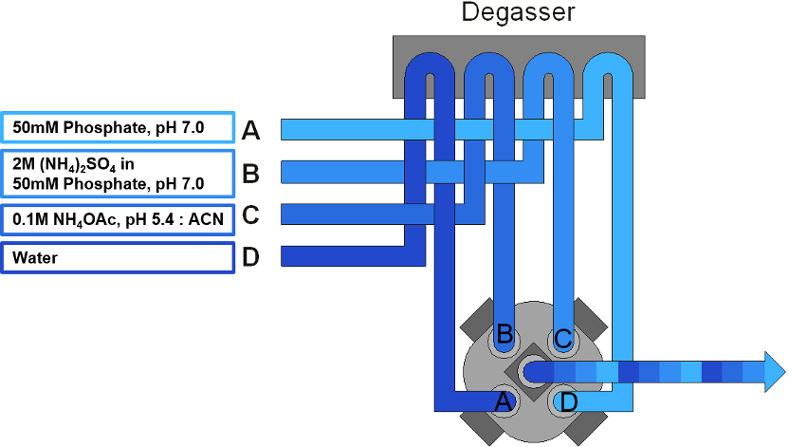
The last tip I have for you today is about the finer details, so bear with me here. If you’re using isopropanol (or any organic solvent), look at the complete arrangement of each solvent like when you designate which line will have which mobile phase. Often, when the lines get to the mixer, some will be arranged above the others – so if the top lines drip or leak, it will fall onto the lower lines. If the upper line has a high salt mobile phase, the line below it should be aqueous rather than organic, so avoid the risk of salt precipitation. If that lower line is water, the salt falls back into aqueous solution and is dissolved. Most of us have it ingrained in our heads that mobile phase A is aqueous and B is organic, and we’re breaking that norm here since both A and B are aqueous. Just double check that wherever your high salt line feeds into the mixer, if there’s a line below it be sure it doesn’t contain organic solvent. Figure 3 shows how these lines are arranged on the Agilent 1260 Infinity II Bio-Inert LC, where we’d want to avoid organic solvent in line A or D.
Figure 3. Solvent line arrangement on the Agilent 1260 Infinity Bio-Inert LC.
That wraps up what I wanted to share with you today. Just remember 1. Store your column appropriately, 2. Check for leaks, 3. Rinse, rinse, rinse, and 4. Be mindful of where the salt is in relation to any organic solvent.
Hydrophobic interaction chromatography was new to me when we started working on this project at Agilent. With a background in mass spectrometry the amount of salt that is used blew my mind! I’ve learned a lot from Greg, Andy, and Sandeep, and hopefully you’ve learned something from them too.
Talk to you soon! - Anne
Keywords: Bio columns, liquid chromatography, hydrophobic interaction chromatography, HIC, tips and tricks, best practices, AdvanceBio blog