Gwen Boone, Norbert Reuter, Global Technical Support for CSD, Middelburg, The Netherlands
Solid Phase Extraction (SPE) is a relatively simple process, but one that requires some thought in order to achieve good recoveries and acceptable cleanliness of your analytes prior to analysis. If you approach optimization methodically in a step-by-step fashion, you will be able to easily improve your recoveries and cleanliness.
By breaking your method into its respective steps instead of tackling it whole will greatly simplify issues.
- Sorbent choice
- Sample Pretreatment
- Conditioning
- Sample Loading
- Rinsing
- Elution

While troubleshooting a new method, it is recommended to collect the eluents generated during the sample addition and the rinse steps, in addition to the elution step. By analyzing these eluents, you can determine where you may be losing analytes. This will provide you with more information concerning which steps need to be optimized in order to increase the recovery.
Sorbent choice
First understand what the analytes are that you are trying to extract versus the contaminants/interferences in your samples. Look for chemical functional groups that are unique to your analyte relative to the interferences. Then, choose a sorbent that can differentiate between your analytes and the interferences via these unique moieties. For example, if your analytes contain an aromatic phenyl group, while the interferences don't, then possibly choose a phenyl phase (i.e. Bond Elut Phenyl).
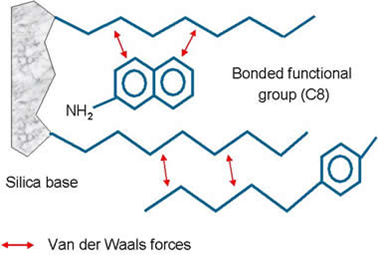
Also consider the sample matrix. If your analyte has a positively charged moiety, but is present in a salt solution, it would not be effective to use a cation exchanger sorbent as you will have competition between your analyte and the other positively charged interferences for the ion-exchange sites.
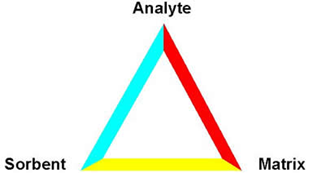
An interrelationship exists between all of these components.
Sample Pretreatment
… is Important. If you will be using a non-polar sorbent, such as Bond Elut C18, and your analyte has a charged group, pretreat your sample by either raising or lowering the pH in order to neutralize this charge. By neutralizing the charge, this will increase the non-polar retention effect for your analyte.
Likewise, if you are using an ion exchange sorbent, adjust the sample's pH in order to ensure that the majority of the analyte will have the appropriate charge for retention.
Conditioning
Conditioning the SPE Cartridge prepares the sorbent phase to accept your pretreated sample. After you condition your cartridge with an initial organic solvent (typically methanol or acetonitrile), follow this up with a aqueous or buffer solution of a similar pH as that of your sample. This will ensure that the analyte encounters the same pH environment upon application to the cartridge and maximizes the retention on the sorbent.
Be sure to avoid drying out the cartridge after conditioning or you will have markedly lower recoveries. You can always repeat the conditioning procedure if you are unsure whether the cartridge has dried out.
Sample Application/Loading
Load your sample using a slow flow rate. If the flow rate is too fast, the force from the vacuum or positive pressure source could be greater than the retention effect exerted by the sorbent (this is especially true for ion exchange sorbents). Instead, load your sample first without pressure turned on, thus allowing the sample to initially percolate into the sorbent. Then, slowly turn on the vacuum or positive pressure to achieve the appropriate flow rate.
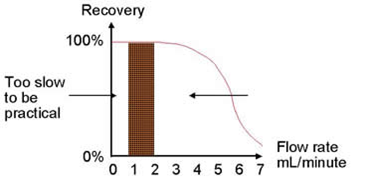
Rinsing
Rinsing (aka Washing) of a cartridge entails a trade-off between cleanliness and recovery. If cleanliness is most important, then some analyte will typically be lost when the cartridge is rinsed with stronger rinsing solvents. If recovery is desired, then the final sample may contain some interferences. Each method should have a consideration as to which is more important, and a balance should be achieved.
When you rinse your cartridges, try a solvent that your analyte is not highly soluble in, but one, which will dissolve the interferences. Use the same pH conditions as when you conditioned your cartridge, thereby maintaining the retention effect between your analytes and the sorbent.
If you are processing serum or plasma samples, try a 10% acetonitrile rinse in order to precipitate any proteins present. Otherwise, when you finally extract the cartridge with a high organic concentration elution solvent, you might notice that the eluent may be clouded with precipitated proteins. Using this low concentration organic rinse will remove these proteins, resulting in cleaner extracts.
Be sure to completely dry your cartridge at this stage. This will ensure complete elimination of any excess rinse solvent, which may possibly still contain interferences. Remember to also wipe the tips of your extraction apparatus for any residual rinse solvent.
Elution
When you elute your analytes from the cartridge, there are several steps that you can take to ensure better recoveries. If you know the general mechanism by which your analyte is being retained by the sorbent, choose a solvent or combination of solvents, which will effectively disrupt these retention mechanisms.
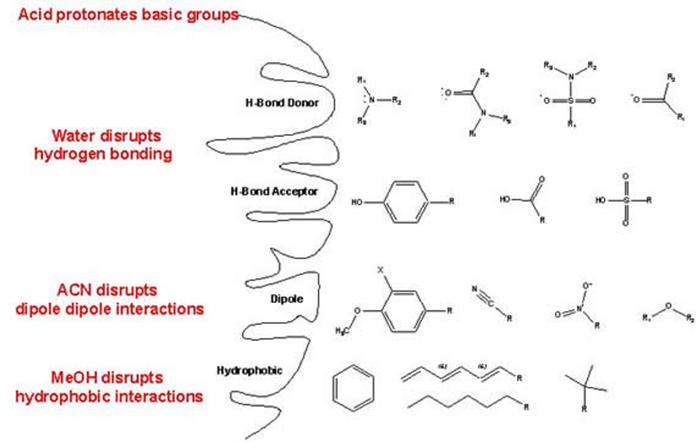
For non-polar extractions:
- Adjust the pH of your elution solvent to ionize your compound, thereby decreasing the retention effect.
- Include an organic modifier, such as methanol or acetonitrile, to disrupt any weak non-polar interaction still existing.
If you are using an ion exchanger there are more options to consider:
- The pH of the elution solvent can be adjusted to either neutralize the charge on the analyte or the ion exchange site (be careful to not exceed the pH constraints of the sorbent).
- A more selective counter-ion can also be introduced to displace the analyte from the ion exchanger site.
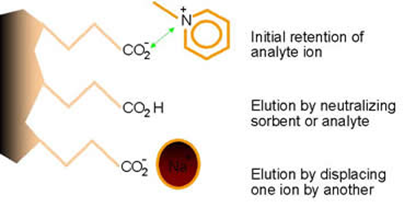
Add the elution solvent in several aliquots. The first aliquot will disrupt the retention effect, while later aliquots will force the solvent (and analytes) through the cartridge. Let the initial aliquot sit in the cartridge a bit before applying vacuum. This will allow it time to disrupt the retention. Use a slower flow rate to allow greater residence time in the cartridge.
