Hi everybody – for the next few weeks I have guest posts from my colleague Wu in R&D. This week he gives some good insight into how to choose the best SEC pore size for your sample. In the next couple of posts he’ll pull back the curtain on a couple of the things R&D considers when designing a column, and how they impact your separation.
Choosing the right pore size for size exclusion chromatography
For successful size exclusion chromatography, it is necessary to choose a column with the right pore size for the separation you are trying to achieve. Molecules that are too large to fit any of the pores will be excluded and elute first. Smaller molecules that can diffuse into the pore structure will take longer to elute from the column, and elute later:
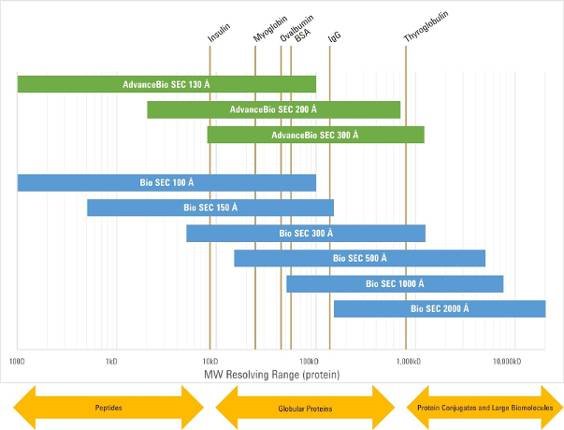
A good rule of thumb is to choose a pore size that is 3x larger than the molecule you are trying to analyze. For a monoclonal antibody (mAb), the size of the molecule is around 5 nm. If you are trying to separate a mAb monomer from its aggregates (dimer, trimer, and higher-order species), the largest molecule could be approaching 10 nm in size. The most appropriate pore size column for this separation is therefore 30 nm, or 300 Å. Alternatively, if you are unsure of the size of your molecule, you may be able to use a column resolving range guide, such as the one below:
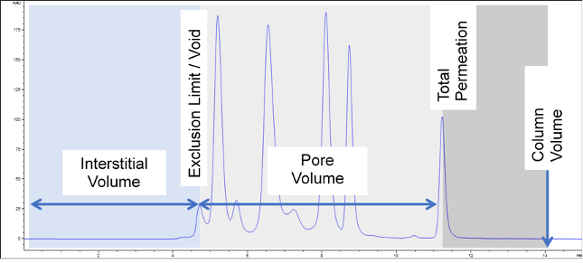
In this case you can see that a mAb of around 150 kDa in size fits in the middle of the resolving range of a 300 Å column. If you are studying a smaller protein such as insulin, you will need to choose a smaller pore size column such as 100 Å or 130 Å. It is important to remember that charts such as this are only a guide. Size exclusion chromatography relies in differences in size in solution, not molecular weight, so molecules that are cylindrical or elongated will elute earlier than expected, as that will have a larger than expected size compared to a compact, globular molecule. Ideally you should choose a column that provides the maximum amount of pore volume and therefore maximum separation ability or resolving power. You can measure the pore volume by looking at the volume of mobile phase eluted between the exclusion point and the total permeation point.
Regularly running a mixture of standard proteins that covers the entire resolving range of the column you are using, like in the chromatogram above, ensures you can keep track of performance and has the added benefit of helping to detect problems early, allowing you to reduce instrument downtime.
Keywords: mAb, monoclonal antibody, ADC, antibody drug conjugates, SEC, size exclusion chromatography, biologics, biosimilars, insulin, molecular weight, aggregation, fragment, quality control, biopharmaceutical, CQA, critical quality attributes