INTRODUCTION
Columns for carbohydrate separations use different chromatographical partitioning techniques, which separate molecules based on a number of different chemical characteristics. The most common mechanism is Ion-Exclusion Partition Chromatography (IEPC) or Ion-Moderated Partition Chromatography (IMPC).
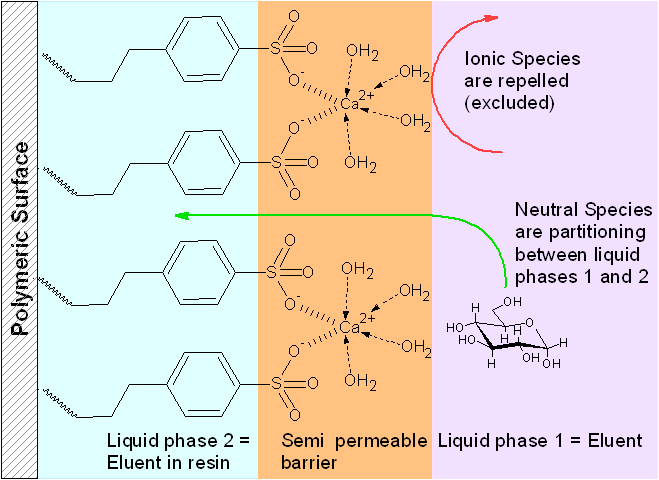
The resin is a polystyrene-divinylbenzene co-polymer with bonded propyl benzenesulfonic acid groups, which can be loaded with a cation (sodium, calcium, lead). The negatively charged sulfonic acid groups and the positively charge metal ions form a kind of semi permeable membrane, which repels ionic species, but is permeable for neutral ones. The eluent in the resin is the second (stationary) phase and neutrals part between the eluent and the "resin-eluent". This partitioning is stimulated for carbohydrates by ligand exchange effects (see below).
Possible interactions within the "resin eluent" are:
- HYDROGEN BONDING
- NORMAL AND/OR REVERSED PHASE INTERACTIONS
- SIZE EXCLUSION EFFECTS
Capability to separate mono-, di- and oligo-saccharides: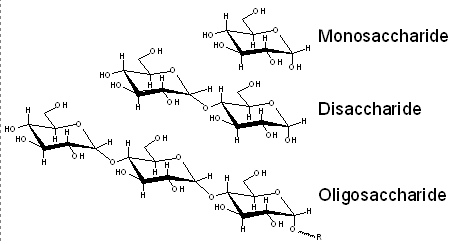
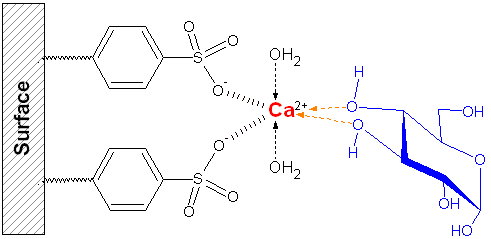
- LIGAND EXCHANGE
COLUMN CHOICE BASED ON APPLICATION
The Hi-Plex product range includes media that meets the USP classification for L17, L19, L34 and L58 and column dimensions as required for the specified methods.
The table below lists the main application areas with the appropriate column recommendations.
| Application Areas | Recommended Column |
|---|---|
| USP methods specifying L17 media | Hi-Plex H |
| USP methods specifying L19 media | Hi-Plex Ca |
| Hi-Plex Ca (Duo) | |
| USP methods specifying L34 media | Hi-Plex Pb |
| USP methods specifying L58 media | Hi-Plex Na |
| Hi-Plex Na (Octo) | |
| Mono-, di-, tri-saccharides | Hi-Plex Ca |
| Hi-Plex Pb | |
| Hi-Plex H | |
| Hi-Plex Na (Octo) | |
| Anomer separations | Hi-Plex Ca |
| Organic acids | Hi-Plex H |
| Alcohols | Hi-Plex Ca |
| Hi-Plex K | |
| Hi-Plex H | |
| Hi-Plex Pb | |
| Adulteration of food and beverages | Hi-Plex Ca |
| Hi-Plex Pb | |
| Food additives | Hi-Plex Ca |
| Hi-Plex Pb | |
| Dairy products | Hi-Plex Ca |
| Hi-Plex H | |
| Sweetened dairy products | Hi-Plex Pb |
| Confectionery | Hi-Plex Ca |
| Hi-Plex Pb | |
| Fruit Juice | Hi-Plex Ca |
| Wine | Hi-Plex H |
| Wood pulp hydrolysates (cellulose/hemi-cellulose) | Hi-Plex Pb |
| Fermentation monitoring | Hi-Plex H |
| Oligosaccharides | Hi-Plex Na |
| Samples with high salt content, (molasses) | Hi-Plex Na (Octo) |
| Oligosaccharides <Dp5 with monosaccharides | Hi-Plex Ca (Duo) |
| Corn syrups | Hi-Plex Na |
RETENTION TIMES OF COMMON CARBOHYDRATES
Where there are several options the retention times of the actual sample components should be compared for each column to identify the columns with the best separation. An aminopropyl column (polar interactions) has been added for comparison reasons.
| Compound |
Retention (mins) |
||||||
|---|---|---|---|---|---|---|---|
| Ca | Ca (Duo) | K | Pb | H | Na (Octo) | Aminopropyl | |
| 300 mm x 7.7 mm | 300 mm x 7.7 mm | 300 mm x 7.7 mm | 300 mm x 7.7 mm | 300 mm x 7.7 mm | 300 mm x 7.7 mm | 250 mm x 4.6 mm | |
| Water with 0.005N Sulfuric Acid | ACN/H2O 3:1 | ||||||
| (0.6 mL/min, 85 ºC) | (0.4 mL/min, 85 ºC) | (0.6 mL/min, 85 ºC) | (0.6 mL/min, 70 ºC) | (0.6 mL/min, 70 ºC) | (0.6 mL/min, 70 ºC) | (1.0 mL/min, ambient) | |
| Adonitol (Ribitol) | 15.2 | 14 | 10.3 | 20.9 | 11.5 | 11 | - |
| Arabinose | 13.7 | 13.7 | 12.7 | 16.7 | 11.4 | 12.4 | 7.5 |
| Arabitol | 17.5 | 15.6 | 10.5 | 30.5 | 11.7 | 11.3 | 7.2 |
| Dulcitol | 19.7 | 16.6 | 10.1 | 45.9 | 11 | 11 | 9 |
| Erythritol | 15.5 | 14.7 | 10.9 | 20.7 | 12.7 | 11.7 | 5.9 |
| Fructose | 13.5 | 13.3 | 11.6 | 19.3 | 10.6 | 11.6 | 8.3 |
| Fucose | 13.8 | 13.7 | 12.5 | 16.7 | 12.2 | 12.3 | - |
| Galactose | 12.3 | 12.4 | 11.5 | 15.3 | 10.7 | 11.3 | 10.3 |
| Glucose | 11.1 | 11.4 | 10.9 | 13.3 | 9.95 | 10.6 | 9.8 |
| Glycerol | 16.1 | 15.5 | 11.8 | 19.6 | 14.1 | 12.7 | not recommended |
| Lactose | 9.68 | 9.77 | 8.6 | 12.1 | 8.5 | 8.58 | 19.5 |
| Maltitol | 12.7 | 11.4 | 8.19 | 27.1 | 8.78 | 8.66 | 15.5 |
| Maltose | 9.34 | 9.59 | 8.54 | 11.7 | 8.4 | 8.5 | 17.4 |
| Maltotriose | 8.46 | 8.76 | 7.55 | 11.1 | 7.7 | 7.61 | 31 |
| Mannitol | 17.4 | 15.1 | 9.96 | 30.7 | 11 | 10.6 | 9.2 |
| Mannose | 12.6 | 12.7 | 11.9 | 20 | 10.5 | 11.4 | 9.1 |
| Melezitose | 8.29 | 8.56 | 7.22 | 9.86 | *8.33 | 7.27 | - |
| Raffinose | 8.46 | 8.66 | 7.31 | 10.4 | *8.2 | 7.38 | 29.7 |
| Rhamnose | 12.7 | 12.7 | 11.2 | 19.8 | 11.6 | 11 | - |
| Sorbitol | 21.5 | 17.2 | 10.3 | - | 11.1 | 11.2 | 9 |
| Stachyose | 7.82 | 8.16 | 6.77 | 9.84 | 7.4 | 6.83 | 67.3 |
| Sucrose | 9.25 | 9.51 | 8.24 | 11 | *9.8 | 8.37 | 14 |
| Xylitol | 20.3 | 17.5 | 10.9 | 42.7 | 11.9 | 11.9 | 7.3 |
| Xylose | 12.1 | 12.5 | 11.8 | 14.4 | 10.6 | 11.5 | - |
| * denotes partial hydrolysis | |||||||
PART NUMBERS
| Product | Crosslink Content | Particle Size | Counter Ion | Column Dimensions | Part Number |
|---|---|---|---|---|---|
| Columns | |||||
| Hi-Plex Ca | 8% | 8 µm | Ca2+ | 300 mm × 7.7 mm | PL1170-6810 |
| Hi-Plex Ca | 8% | 8 µm | Ca2+ | 250 mm × 4.6 mm | PL1570-6810 |
| Hi-Plex Ca (Duo) | 8% | 8 µm | Ca2+ | 300 mm × 6.5 mm | PL1F70-6850 |
| Hi-Plex Ca USP L19 | 8% | 8 µm | Ca2+ | 250 mm × 4.0 mm | PL1570-5810 |
| Hi-Plex Pb | 8% | 8 µm | Pb2+ | 300 mm × 7.7 mm | PL1170-6820 |
| Hi-Plex Pb USP L34 | 8% | 8 µm | Pb2+ | 100 mm × 7.7 mm | PL1170-2820 |
| Hi-Plex K | 8% | 8 µm | K+ | 300 mm × 7.7 mm | PL1170-6860 |
| Hi-Plex H | 8% | 8 µm | H+ | 300 mm × 7.7 mm | PL1170-6830 |
| Hi-Plex H | 8% | 8 µm | H+ | 300 mm × 6.5 mm | PL1F70-6830 |
| Hi-Plex H | 8% | 8 µm | H+ | 220 mm × 4.6 mm | PL12506 |
| Hi-Plex H USP L17 | 8% | 8 µm | H+ | 100 mm × 7.7 mm | PL1170-2823 |
| Hi-Plex Na | 4% | 10 µm | Na+ | 250 mm × 4.6 mm | PL1571-6140 |
| Hi-Plex Na | 4% | 10 µm | Na+ | 300 mm × 7.7 mm | PL1171-6140 |
| Hi-Plex Na (Octo) | 8% | 8 µm | Na+ | 300 mm × 7.7 mm | PL1170-6840 |
| Guard Columns | |||||
| Hi-Plex Ca | 8% | 8 µm | Ca2+ | 50 mm × 7.7 mm | PL1170-1810 |
| Hi-Plex Ca (Duo) | 8% | 8 µm | Ca2+ | 50 mm × 7.7 mm | PL1170-1850 |
| Hi-Plex Pb | 8% | 8 µm | Pb2+ | 50 mm × 7.7 mm | PL1170-1820 |
| Hi-Plex K | 8% | 8 µm | K+ | 50 mm × 7.7 mm | PL1170-1860 |
| Hi-Plex H | 8% | 8 µm | H+ | 50 mm × 7.7 mm | PL1170-1830 |
| Hi-Plex Na | 4% | 10 µm | Na+ | 50 mm × 7.7 mm | PL1171-1140 |
| Hi-Plex Na (Octo) | 8% | 8 µm | Na+ | 50 mm × 7.7 mm | PL1170-1840 |
| Guard Cartridges | |||||
| Hi-Plex Ca | 8% | 8 µm | Ca2+ | 5 mm × 3 mm (2) | PL1670-0810 |
| Hi-Plex Ca (Duo) | 8% | 8 µm | Ca2+ | 5 mm × 3 mm (2) | PL1670-0850 |
| Hi-Plex Pb | 8% | 8 µm | Pb2+ | 5 mm × 3 mm (2) | PL1670-0820 |
| Hi-Plex K | 8% | 8 µm | K+ | 5 mm × 3 mm (2) | PL1670-0860 |
| Hi-Plex H | 8% | 8 µm | H+ | 5 mm × 3 mm (2) | PL1670-0830 |
| Hi-Plex Na | 4% | 10 µm | Na+ | 5 mm × 3 mm (2) | PL1671-0140 |
| Hi-Plex Na (Octo) | 8% | 8 µm | Na+ | 5 mm × 3 mm (2) | PL1670-0840 |
FREQUENTLY ASKED QUESTIONS
Q. How do I choose between the 4% and 8% cross linked media?
A. The difference is in the pore size of the material, the lower the crosslink density the bigger the pore size is, so the 4% material is used for oligosaccharide analysis and the 8% for oligosaccharide <dp 3.
Q. How do I choose the most appropriate counter ion for the analysis?
A. The resin must be selected based on the solute of interest – refer to the table of elution times to identify the most effective column.
Q. Can I change the counter ion on the Hi-Plex column?
A. Because the Hi-Plex materials are microporous the swell will change with the counter ion – therefore if the counter ion is exchanged on a pre-packed column swelling or shrinking will occur and the column will either over pressure or void – these materials are termed fixed-ion exchangers.
Q. Can I use eluents other than water with the Hi-Plex columns?
A. It is important to ensure that the counter ions are not stripped from the Hi-Plex columns so eluent containing cations that are different to those on the media should never be used – however, with the Hi-Plex Na it is possible to run at high pH with sodium hydroxide as the eluent and the Hi-Plex H can be used with acid eluents.
Q. To protect my analytical column, should I use a guard cartridge or a guard column?
A. This depends on the sample matrix – the cartridges have a small volume of 35 µL. The interstitial volume (remember: interstitial volume = volume of empty column tubing - volume of packing) is ca. 14 µL, so the capacity is low and they will quickly foul and should not be used if the sample contains high concentration of salts or any concentration of a salt with a metal ion that is stronger than that bound to the phase (see counter-ion selectivity's).
Q. Why do I have to run my column at lower flow rate when first installed on the HPLC?
A. As the Hi-Plex media is microporous, the columns are packed at lower pressures than silica based HPLC columns so when installed it is important not to exceed the maximum operating pressure. Water is viscous and the pressure is high at room temperature but as the temperature increases so viscosity reduces and the flow rate can be increased.
Q. How should I remove the Hi-Plex column from the HPLC instrument?
A. As the column will have been operated at high temperature – 60 °C to 95 °C it must be allowed to gradually cool to room temperature – switch off the oven and let the thermal mass of steel cool. While the column is being decommissioned, the eluent flow should be reduced to 0.2 to 0.1 mL/min
Q. How do I store the column?
A. Once the column is at room temperature, the end caps should be replaced and the column stored at 4 °C or in a cool lab. The columns should be stored in water without sodium azide.
Q. The column has lost performance – can it be regenerated?
A. There are two main reasons why a column will fail, the maximum operating pressure has been exceeded and it has voided or the counter ions have been stripped. If the column has voided then it cannot be regenerated, but if the counter ions have been stripped the regeneration procedure detailed in the Column User Guide should be followed.
Q. My sample has no UV chromophore, what detector should I use?
A. As the Hi-Plex separations are isocratic it is possible to use a refractive index detector, such as the Agilent 356-LC Refractive Index Detector. If more sensitivity is required then an ELSD can be used, Agilent 385-LC ELSD or Agilent 380-LC ELSD.
