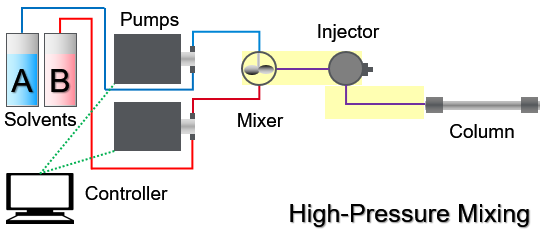
The dwell volume in Liquid Chromatography, LC, is the volume from the mixer to the column head. In a two-pump system with high-pressure mixing the dwell volume is marked with the yellow box:
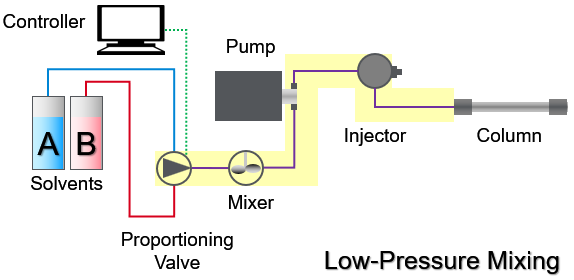
In a single pump low-pressure mixing system with proportioning valve it is different and marked by the yellow box:
In isocratic runs, the dwell volume has no influence on the separation.
In gradient systems, there is a significant influence when transferring or translating a method to another column or system. At least this is the case when the dwell volumes of the two systems are different. Keep in mind the dwell volume is an extra dead volume and has an influence on when a gradient change arrives at the column head. The larger a dwell volume is, the longer it will take to arrive at the column head.
The Two Possible Cases:
- The dwell volume of system 1 is greater than the dwell volume of system 2:
In this case, the gradient change arrives at the column head of system 2 earlier than expected. To get a similar chromatogram than on system 1 you must add an extra isocratic hold time at the beginning of the analytical run to compensate for this effect. - The dwell volume of system 1 is less than the dwell volume of system 2:
In this case, the gradient change arrives at the column head of system 2 later than expected. To get a similar chromatogram than on system 1 you must start your run first without injection, after the necessary compensation time (pre-injection time) you inject.
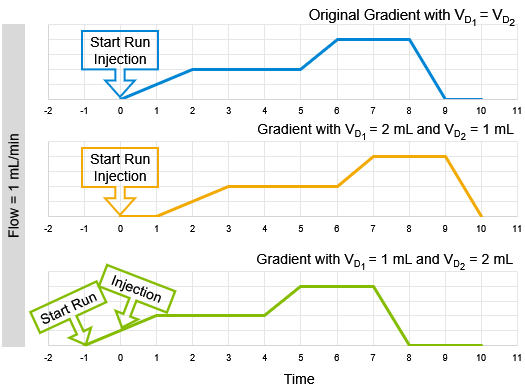
Here is a graphical example how the original gradient needs to be adjusted when the dwell volumes change. Flow = 1 mL/min and the dwell volumes 1 or 2 mL to keep it simple:
Blue is the original gradient, orange the one with an isocratic hold time (dwell volume 1 > dwell volume 2) and green is the one with pre-injection time (dwell volume 1 < dwell volume 2). All three gradients in the three systems would show the same chromatogram.
Determining the dwell volume
For this calculation, the column in the system needs to be replaced by a very low volume tubing, preferably with an internal volume of 15 µL or less. Solvent A is water and solvent B is water with 0.1 % acetone or any other UV-absorbing solvent. Acetone has an UV absorption at 265 nm and the detector will see the increasing amount of acetone easily.
Start a gradient from 100 % A to 100 % B in any time (gradient time, tGradient) and record the chromatogram.
- Method 1:
Draw a tangential line through the inflection point of your chromatogram, the time where this line intersects with the base signal line is the dwell time.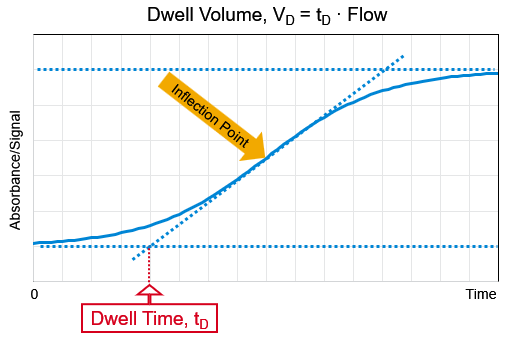
- Method 2:
The gradient time is known for the run. Half the distance between the high and the low baseline. From the 50% height point let a line parallel to the baseline intersect the chromatogram. The time of this intersection point is t50%. The dwell time is t50% - 0.5 · tGradient.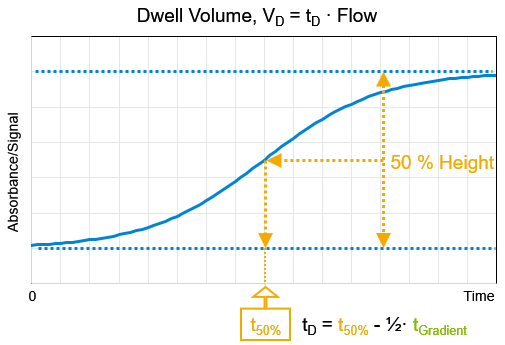
For both methods, the dwell time is multiplied by the flow to get the dwell volume.
Dwell volume vs. dead volume
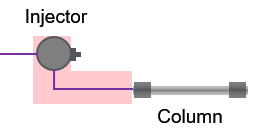
Don't confuse dead volume with dwell volume. The dead volume is only part of it—the volume from the injector to the column head.
References
- "Measure the dwell volume", European Pharmacopoeia 7.0, 2.2.46.