Eric de Witte, Norbert Reuter*, Global Technical Support for CSD, Middelburg, The Netherlands
For 20 years already the use of smaller internal diameter analytical HPLC columns has been discussed. Why is this so interesting and what are the advantages and/or disadvantages of smaller ID columns? Some considerations.
The early 80‘s saw the introduction of smaller internal diameter HPLC columns. The nomenclature of small ID columns is not always clear and terms like microbore, minibore, and capillary are used indifferently.
The definition can be as follows.
|
Standard columns |
are referred to as columns with an ID of |
4.6 mm |
|
Minibore columns |
2.0 mm |
|
|
Microbore columns |
1.0 mm |
|
|
Capillary columns |
0.32 mm |
Besides a number of attractive advantages when going to smaller ID columns, like lower solvent consumption, enhanced sensitivity for mass limited samples and compatibility with selective detectors requiring low solvent use (LC/MS), there are also a number of technical, mainly instrumental problems, which needed to be solved.
All the technical problems have been solved since. However, the advantages of microbore and minibore columns vs. standard HPLC columns are too small to initiate a breakthrough in the application of these types of columns in routine HPLC laboratories yet.
A comparison table can also be found in the appendix.
Solvent consumption:
Comparing a 250 mm x 4.6 mm column vs. a 250 mm x 2.0 mm column with identical packing material and particle size, the optimum linear velocity of the mobile phase is the same.
The internal volume of a 2.0 mm ID column is however 5 times smaller compared to a 4.6 mm ID column. This means that when both columns are used at their optimum flow rate, the flow rate for the 2.0 mm column will be 5 times lower compared to the 4.6 mm ID column, while retention times will remain the same. This results in a 5 times lower solvent consumption for 2.0 mm internal diameter columns vs. 4.6 mm columns. For 1.0 mm ID columns the solvent reduction is 20 fold.
So, by using smaller ID columns, a substantial reduction in solvent consumption can be achieved, reducing costs and cutting down on environmental pollution.
Enhanced sensitivity for mass limited samples
Most LC detectors such as UV, FLD and MS are concentration dependent. This means that the maximum peak height for a particular component is directly related to the maximum concentration (Cmax) of the solute in the detector cell.
The dilution of the sample in the column during the chromatographic process will reduce its detectability.
[EQ. 1] 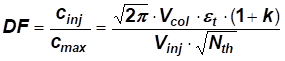
DF = dilution factor
cinj = sample concentration
cmax = concentration in the detector
Vcol = column volume
Vinj = injection volume
et = total column porosity
k = retention factor
Nth = theoretical plate number
As can be seen from equation 1 the dilution factor is directly proportional to the volume of the column (Vcol)
Assuming that all parameters remain unchanged and columns are used at their optimal flow rate, 2.0 mm ID columns will therefore give 5 times less sample dilution in the column compared to 4.6 mm ID columns and as a result will generate roughly 5 times higher peaks.
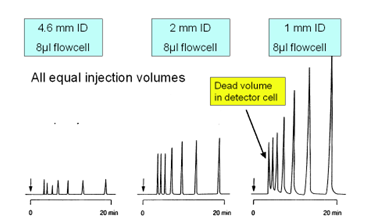
Figure 1: comparison between different ID columns.
A 1.0 mm ID column gives an even higher sensitivity than 2.0 mm ID columns but due to the instrumental limitations (contribution to band broadening in the detector) it quickly will cause high dead volumes and as a consequence peak tailing in the first part of the chromatogram.
However, this is only the part of the story. Dilution of the sample is one issue but in practice it is possible to inject a higher volume on a 4.6 mm ID column then on a 2.0 mm ID column. In practice it means there is no real gain in sensitivity if enough sample can be injected.
However, these columns are ideal for very sample-limited applications
Compatibility with selective detectors requiring low solvent use (LC/MS)
The lower flow rates, which are used for smaller ID columns, also make the on-line coupling with other techniques like LC-GC and LC-MS more feasible.
The new generation interfaces and API techniques allow the use of a wide range of column flows into the MS system. It can range from a few µl/min to the more conventional flows of 1 - 2 ml/min. Columns with standard internal diameters of 4.6 mm can therefore be used. However, modern LC-MS equipment often shows its best performance with respect to sensitivity, background and noise level at flows rate of 0.2 – 0.4ml/min. This is exactly the optimal flow rate for columns with an ID of 2.0 mm.
Instrumental problems
The overall measured peak width / variance (σ²overall) or total band broadening does not only originate from the column. There is also a significant contribution from the injector, detector, tubing and fittings as expressed in equation 2.
[EQ. 2] 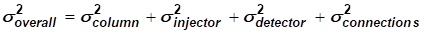
or
[EQ. 3] 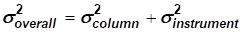
Of course the column band broadening variance must be kept small, but the contribution of the instrument should even be smaller. Acceptable separation and peak shape, especially for early elution peaks, can be achieved in practice if σ²instrument < 0.1 σ²column
Using the definition of the column theoretical plate number gives us the following relation for the eluting peak volumes
[EQ. 4] 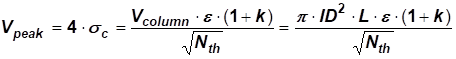
As can be seen from equation 4, column band broadening (peak volume) is directly related to the column volume, the smaller the column volume the smaller the peak volume. It is clear from equation 3 and 4 that the external column band broadening becomes very critical with decreasing column volume (diameter). This is even more relevant for early eluting peaks (low k values), because the lower the k-value the lower the peak volume
A well-designed standard HPLC system (injection volume 30 µl, detector cell volume 15 - 30 µl) may tolerate column peak volumes as low as 110 µL and still provide good efficiency. An early elution peak (k = 1) on a standard column has a peak volume of about 200 µl.
Early eluting peaks on a 2.0 mm ID column will show extra peak broadening on a standard system, because the peak volume will be about 40 µl or lower. These peak volumes will require a careful design of the HPLC system, so that the possibilities of the small-bore columns may be fully utilized. This means that the injection volume has a maximum of 5 µl. Detector cell volume needs to be 5 µl or lower. The internal diameter of the connecting tubing between injector and pump must be 0.15 mm or less.
Another important factor to consider is the flow rate, which the pump can deliver. Most HPLC systems are designed to provide optimum flow precision between 0.2 and 5ml/min. When using HPLC columns with internal diameters less than 2.0mm these HPLC systems are often not capable of delivering flow rates with enough precision to obtain reproducible chromatography.
So, when is it profitably to use these small-bore columns? First you have to consider whether the HPLC system allows the efficient use of a small bore column. If the answer is yes, there is no reason not to use 2.0mm ID columns. You can benefit from the lower solvent use (cost reduction) and in case of limited sample amount you also benefit from the increased mass sensitivity
Appendix
Comparison Table
|
|
Standard column |
Minibore Column |
Microbore column |
|
Column dimensions L x ID (mm x mm) |
250 x 4.6 |
250 x 2.0 |
250 x 1.0 |
|
Column Volume (mL) |
2.5 |
0.5 |
0.1 |
|
Particle size (μm) Packing material |
5 |
5 |
5 |
|
Plate number |
18000 |
18000 * |
18000 * |
|
Optimum flow rate (mL/min) |
1.0 |
0.2 |
0.05 |
|
Retention time (min) |
10 |
10 |
10 |
|
Solvent use (mL/analysis) |
10 |
2 |
0.5 |
|
Relative peak height |
1 |
5 |
20 |
|
Max detector cell volume (μL) |
15 – 30 |
2 – 10 ** |
2 ** |
|
Max injection volume (μL) |
30 |
5 |
1 |
|
Peak Volume (μL) |
|
|
|
* These plate numbers are theoretical values. In practice the plate number for the 2.0 mm and especially for the 1.0 mm columns
will be lower, compared to a 4.6 mm column, mainly due to wall effects.
** It is not only the detector cell volume, which matters, but also the mechanical construction (flow profile) of the cell, which
determines the extra peak broadening. Sometime better results are obtained with a well-configured higher volume flow cell.
The advantage of a higher flow cell volume is the longer path length and hence higher sensitivity
Click here for the LC Method Translator App
