 Hi
Hi
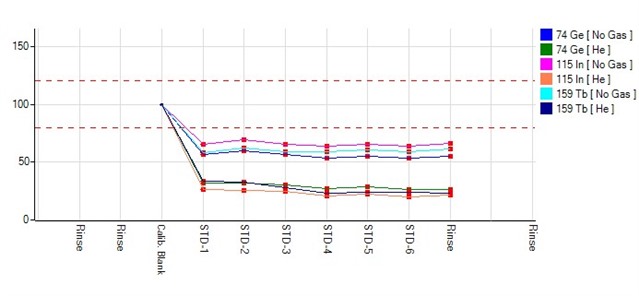
The internal standard recovery on ICPMS 7900 recently falls down right after calibration blank. All the controls and references are OK but the recovery percentage is too low, 20-30%, does anybody had the similar problem? The concentration is as before.
Thank
