Hello,
I am having a very strange problem with some analytes I am dealing with. The system I am using is an Agilent 8890 GC with a 7000D mass spectrometer. The injector is from Gerstel and consists of a thermal desorption unit (TDU2) and a cooled injection system (CIS) which is basically a PTV inlet. The column is a StabilWax-MS from Restek. The sample types are foods and we are analyzing flavor and fragrance compounds.
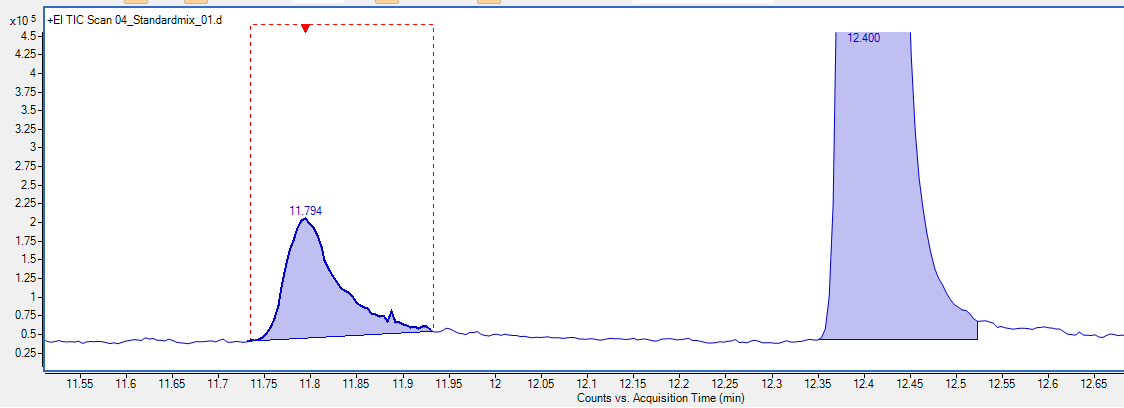
While running a variety of samples I noticed peaks doubling. Some analytes were showing up twice at about ~0.5min-1min apart. Here are some images that illustrate the problem in a standard mixture.
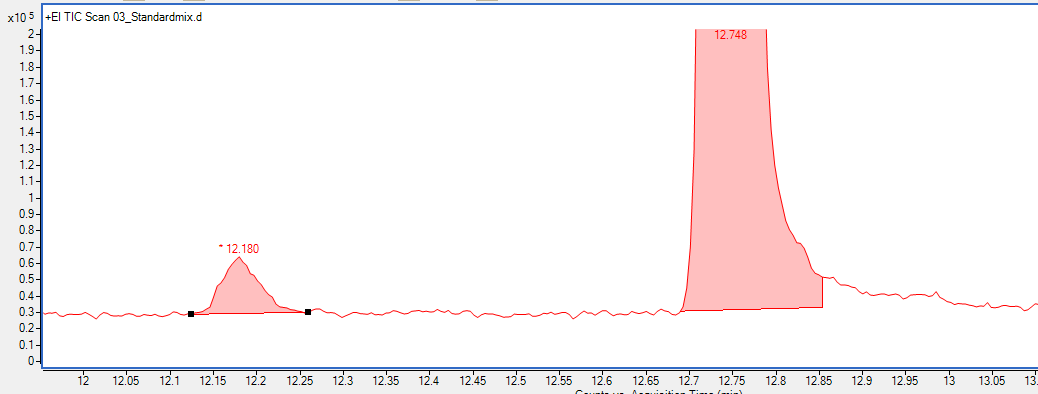
Here is the standard mix.
Peaks look normal except if you look closely two of the peaks are 'doubling'. Specifically 2-methylpyrazine and 2-heptanol.
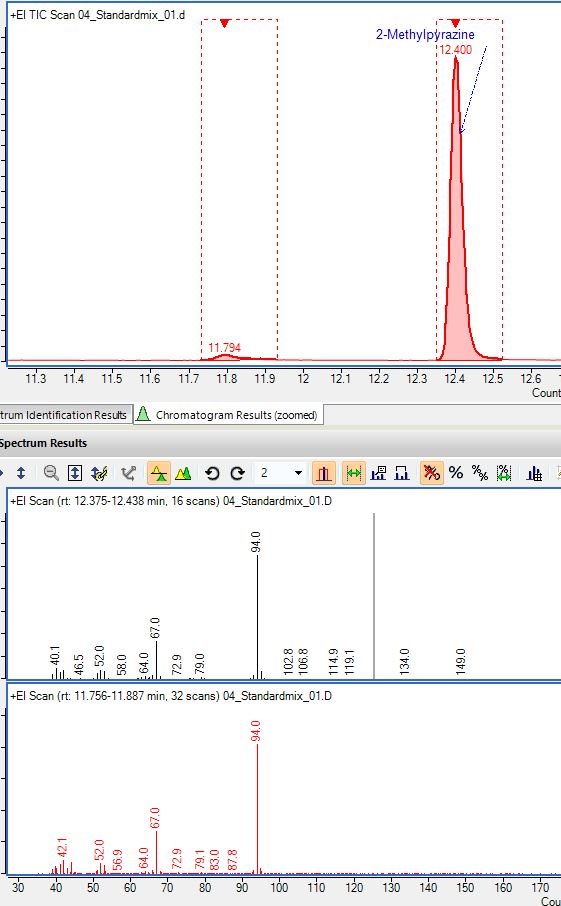
These compounds didn't do this before and this problem only started happening after I had evidence of a contaminated inlet/column. I changed liner and problem went away temporarily but came back after running some samples and then checking standards again. Clipping column similar issue. So I replaced the whole column and I am in process of testing if that was the problem. Has anyone ever seen anything like this before? I don't have a lot of experience with Wax type columns but this behavior is something I've never seen before. It could be an issue with TDU/CIS and I'll know more after testing new column. Its just so strange to only be occurring with certain compounds. I was seeing a similar phenomenon with real samples and it was making data processing extremely confusing.