I am isolating mitochondria from mouse brain and want to run seahorse onto it, could you recommend the best possible protocol for the isolated mitochondria on seahorse XFe96 analyzer?
Looking forward,
Best regards.
Gaurav
I am isolating mitochondria from mouse brain and want to run seahorse onto it, could you recommend the best possible protocol for the isolated mitochondria on seahorse XFe96 analyzer?
Looking forward,
Best regards.
Gaurav
Hi Guarav,
Thank you for contacting Cell Analysis Technical Support. We do have some resources/protocols regarding Iso Mito on the 96 platform:
Analyzing Microgram Quantities of Isolated Mitochondria in the Agilent Seahorse XFe/XF96 Analyzer
Keep in mind that the isolation of mitochondria itself will differ from species to species and cell type to cell type, so you will have to find the best method specific to mouse brain mito.
Please let me know if these resources help or you have any other specific questions!
Thank you,
Courtney
Courtney Nadeau Watts
Technical Support Scientist/Remote Engineer
Cell Analysis Products
Phone Contact: 800-227-9770 (Option 3 , option 8)
Email Contact: cellanalysis.support@agilent.com
Thanks Courtney,
I went through the recommended papers, and it helped me to understand better. What I could not find that what combination of substrate I need to use on my isolated mitochondria on seahorse plate to keep them respiring? I am using seahorse assay buffer (DMEM+Sod. Pyru+Gluc+Glutamine) for the cells to be used in the assay.
My set-ups are:
Port-A: ADP (Different concentration from 0.5 to 4 mM)
Port-B: Oligo
Port-C: FccP
Port-D: Rot+Antimycin
I am using primary neurons from the mice to isolate the mitochondria.
Let me know!
Best,
Gaurav
Hi Guarav,
Great, I am glad those helped! We do have a few updates that we now suggest. These will be released at some point in written form, but please see below for the time being. Regarding the solution that you should be using during the assay: this will be 1X MAS - see screenshot below. This assay does not use Seahorse media at all as iso mito rather than intact cells are being used.

Regarding the combination of substrates/inhibitors needed, this will depend on which complexes you are interested in testing and/or what question(s) you are trying to answer with your research. I think the below chart from this paper will be helpful. This paper relates to PMP, but there is some overlap between PMP and iso mito.
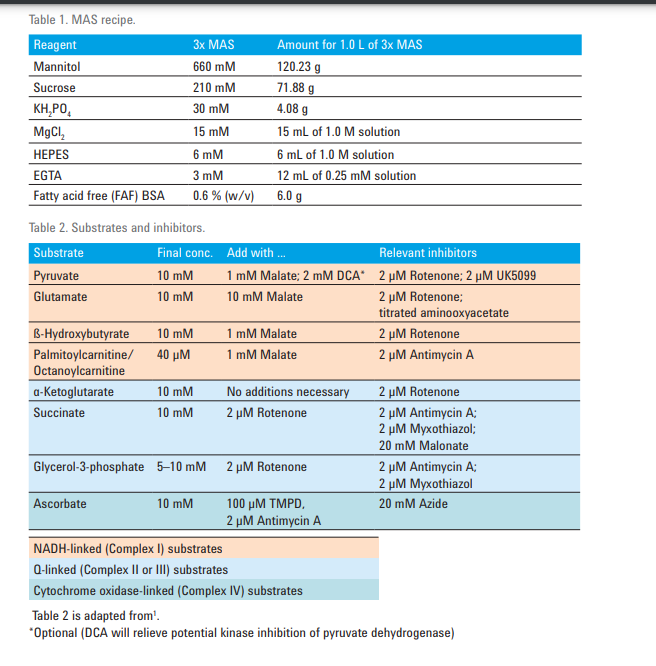
I also want to make sure you are aware that the Agilent Mito Stress Test is not applicable to iso mito either as this assay kit was designed for intact cells, and they concentrations in the kit's reagents will not be sufficient for iso mito.
Thank you and please let me know if this helps or you have any other questions!
Courtney
Dear Courtney,
Thank you for your reply. I read the paper you attached to answer my question. But I was not aware that Mito Stress kit was not compatible with Isolated mitochondria and ofcourse my experiment did not work.
I am isolating mitochondria from neuron cells and I want to see whether isolated mitochondria are metabolically active and respiration competent. I will be using this mitochondria for transplantation later on with initially co-culture and later on in in-vivo. Below is the link of the protocol we used from Boston hospital, USA and it's a rapid protocol to isolate mitochondria.
Could you suggest me what experiment I should opt for on Seahorse to analyze my isolated mitochondria?
I look forward to hearing from you.
Best Regards,
Hi Gyarav,
You are very welcome. The assays we have in this paper are Coupling or Electron Flow. The Coupling Assay examines the degree of coupling between the electron transport chain (ETC), and the oxidative phosphorylation machinery (OXPHOS), and can distinguish between ETC and OXPHOS with respect to mitochondrial function/dysfunction. The Electron Flow assay examines sequential electron flow through different complexes of the electron transport chain, which can identify the mechanism of mitochondrial dysfunction or modulation. The assay choice will be up to your discretion.
Please let me know if this helps.
Thank you,
Courtney
Dear Courtney,
I would like to know is there have Finished kit for Coupling or Electron Flow measurement in isolated mitochondrial with XFe24. Could you recommend any appropriate reference materials? If I'm using a commercial mitochondrial isolation kit for isolating mitochondria, is it correct that I only use the MAS buffer for resuspending the mitochondria in the final step?
I look forward to hearing from you.
Best Regards,
LD
Hi LD,
Thank you for contacting Cell Analysis Technical Support. Unfortunately, we do not have a kit for Coupling or Electron Flow, but the below paper does a great job with reference materials, including part numbers and manufacturer.
Regarding when to add the MAS buffer, please see below from our Iso Mito App Note:
"XF Assay Preparation To minimize variability between wells, mitochondria were first diluted 10x in cold 1x MAS + substrate, then subsequently diluted to the needed concentration required for plating. Note that substrates included in the initial dilution, and is present during the centrifugation step."
Thank you and please let me know if you have any other questions!
Courtney
Courtney Nadeau Watts
Technical Support Scientist/Remote Engineer
Cell Analysis Products
Phone Contact: 800-227-9770 (Option 3 , option 8)
Email Contact: cellanalysis.support@agilent.com
Hi Courtney,
Thank you for your reply. Your reply has cleared up many of my doubts.
Different tissues may have different preferences for metabolic substrates, so are there any guidelines for the metabolic substrates of mitochondria sourced from different tissues for use in Seahorse assays? For instance, mitochondrial substrates from cardiac tissue or skeletal muscle tissue.
I look forward to hearing from you.
Best Regards,
LD
Hi LD,
You are very welcome! Table 25.2.1 in the paper above gives recommendations. You can also take a look at our Cell Publication Database to see what others have done as far as this.
Thank you and let me know if you have any other questions.
Courtney
Hi Courtney,
Thank you for your response. I have a question regarding the selection of the solvent for the respiration reagent. I've encountered conflicting information on this matter. The literature (PMID: 35771443) indicates the use of DMSO, whereas the information provided to me suggests a solution in 95% ethanol. Could you please advise me on which option to choose?
I eagerly await your response.
Best Regards,
LD
Hi LD,
You are very welcome! Our protocol recommends using 95% ethanol (do not use 100 % ethanol as it contains traces of benzene, which is detrimental to mitochondrial function) rather than DMSO. Also be sure not to add ethanol to the injection ports as this can cause leaks/injection issues in your assay.
Thank you and let me know if you have any other questions.
Courtney
Hi LD,
You are very welcome! Our protocol recommends using 95% ethanol (do not use 100 % ethanol as it contains traces of benzene, which is detrimental to mitochondrial function) rather than DMSO. Also be sure not to add ethanol to the injection ports as this can cause leaks/injection issues in your assay.
Thank you and let me know if you have any other questions.
Courtney
Hi Courtney,
Thank you for your response. I have a question about the Mitochondrial Assay Solution (MAS, 1X). When conducting Seahorse experiments with isolated mitochondria, is it acceptable to substitute EDTA for EGTA when preparing the Mitochondrial Assay Solution (MAS, 1X)?
I eagerly await your response.
Best Regards,
LD
Hi LD,
We do not recommending substituting as EGTA has higher affinity for chelating calcium ions and lower affinity for chelating Mg2+ ions compared to EDTA.
Thank you and please let me know if you have any other questions!
Courtney