Hello,
We're using Agilent articles 5991-9507EN, 5994-5561EN, and 5994-5149EN to develop a method for the analysis of calcium and other metals in lithium carbonate. We prepare high TDS samples by the dissolution of Li2CO3 with concentrated nitric acid then dilution with 2% nitric acid.
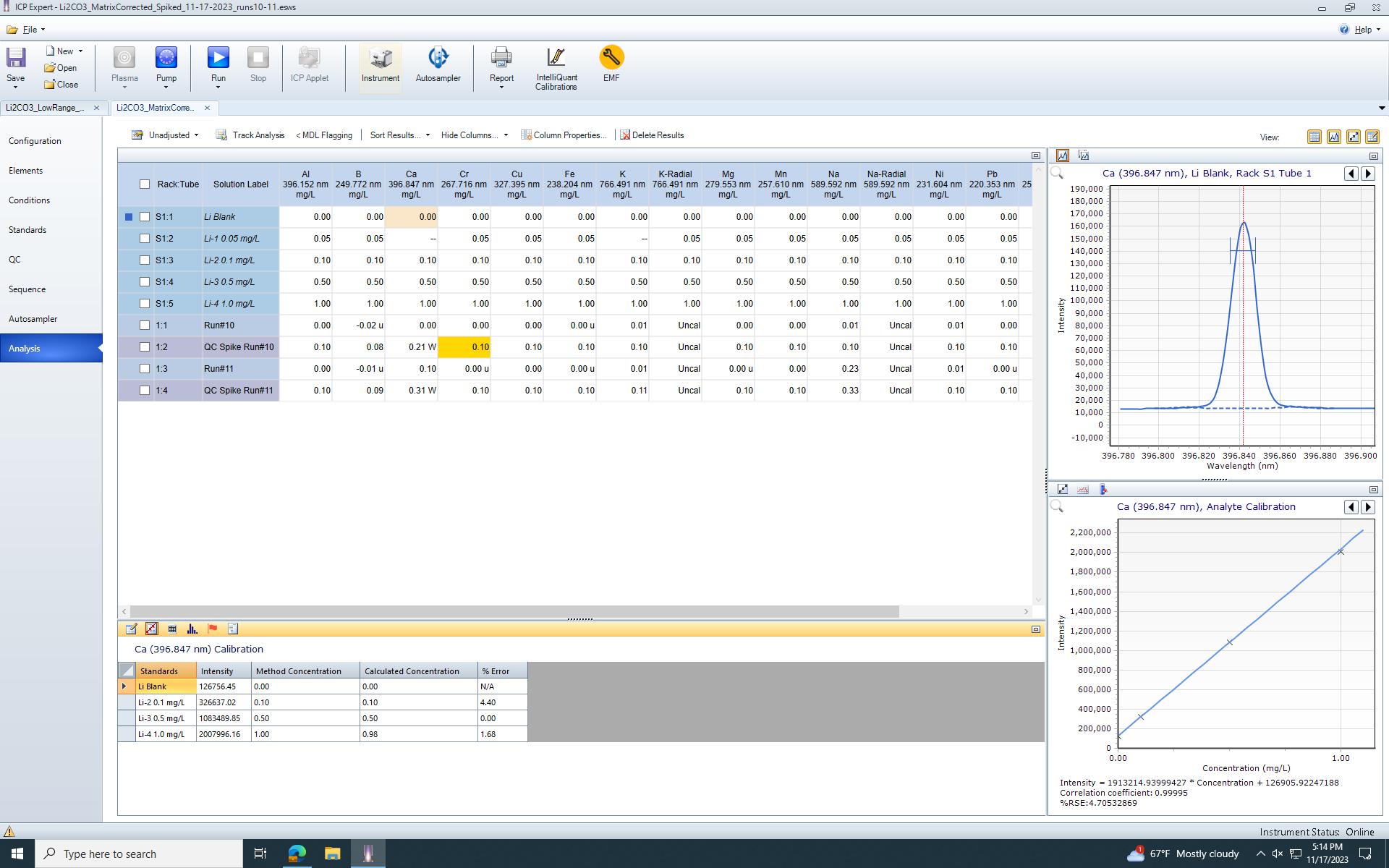
To correct for the High-TDS samples, we matrix match by addition an aliquot of high purity lithium solution to each one of our calibration samples to build a standard curve of 0.1ppm, 0.5ppm, 1.0ppm for all of our elements of interest. Additionally, we are running everything with a continuously supplied internal standard (Y (371.029 nm)). We also are using the QC portion of the software to run QC Spikes to verify the accuracy of the method. Our QC spikes are at 0.1ppm.
The issue we are running into is that the lithium solution we use to matrix match has a similar amount of calcium as some of our samples, so measurements on the low-end of the concentration curve become innacurate. Our QC spike shows concentrations of 0.3 ppm in the solution instead of the 0.1ppm it was spiked with (All other elements, including Na, K, etc, have reasonable and acceptable spike recoveries). I've remade the standard curve several times as well as remade the sample dilutions to be certain that it isn't contamination, and I don't think that it is.
I have not found anywhere in the software where I can subtract the blank from the calibration curve. The QCBLK function appears to only subtract the blank from the samples, when it is my standard curve that is contaminated. There is no way to avoid this contamination in the standard curve.
Is it possible in the ICP expert software to subtract the blank from the calibration, not just to not include the calibration?
Is there a way to do this analysis that isn't the method of standard additions?
(Screenshot of current analysis, with samples and QCSPK samples)
i