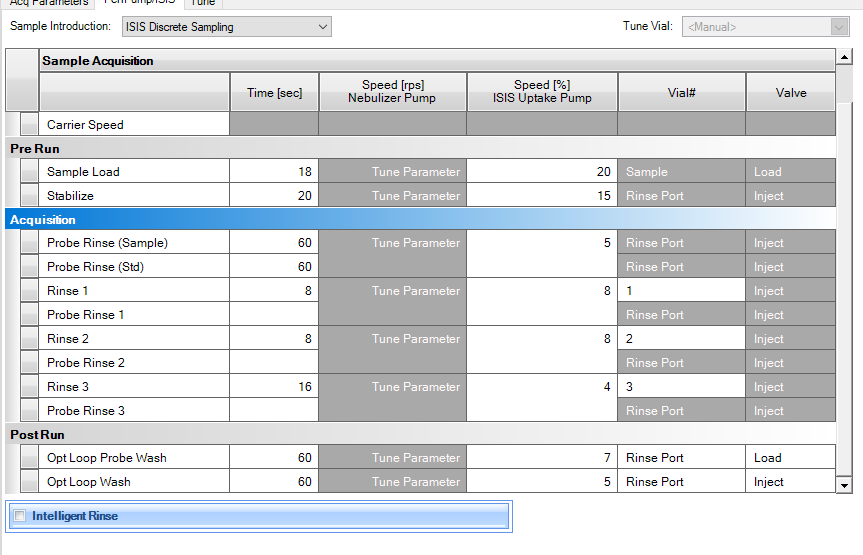
I run a 7800 doing EPA 200.8 MET and 6020B methods. Recently, we've had an instrument serviced and have been seeing Mo and Se carryover into ICB and CCBs at levels > ½RL.
Here are the things I've tried to address this:
- Loaded an old batch and tune from before the PM
- Replaced the nebulizer
- Cleaned the rotary valve
- Replaced the spray chamber
- Cleaned the torch
- Replaced cones
- Dramatically increased rinse time by a minute
So far, no luck. I'm suspecting it's something in the software, like some tune settings or something like that.
Does anyone have any ideas on what it could be?
Update:
After doing some research I've come to the conclusion that the issue is the switching valve. When it switches over to "inject" the Mo comes through. It seems the inside isn't being cleaned well enough to.
- Replaced both the stator and rotor
- Cleaned the heck out of the valve
No matter how long I have my rinses (within reason) it still carries over.