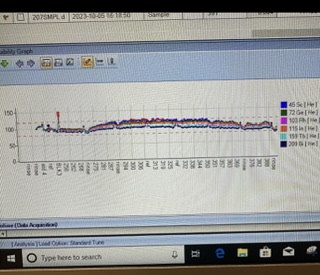
I am very frustrated about the ISTD recovery ratio keeps going up, I run total around 150 animal tissue samples, I treated samples so matrix all match, but after 70 samples the ISTD keeps going up to 120% and data shows abnormal from ref QC. Machine performance good, Tune good, everything looks good at beginning but all of sudden it goes crazy. Could you give me some idea what is going on? Sometime it it goes down to 75%. Thank you.