Hello All,
I am experiencing a huge baseline increase issue during my plasma metabolome analysis exp.
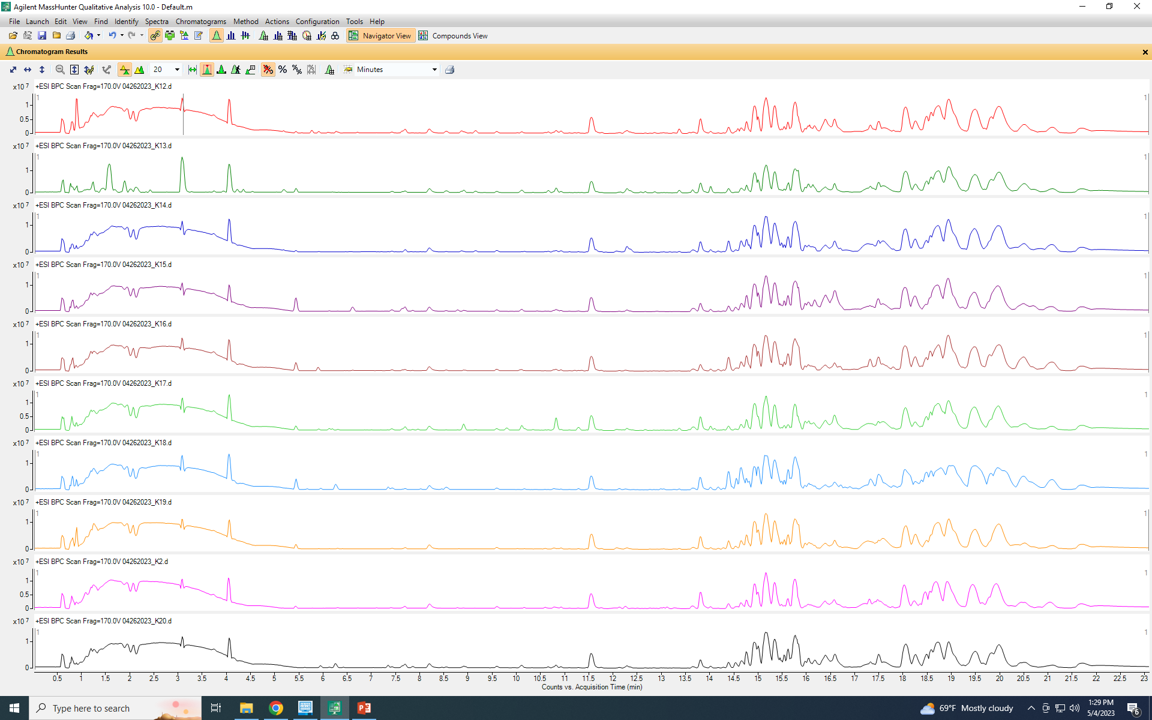
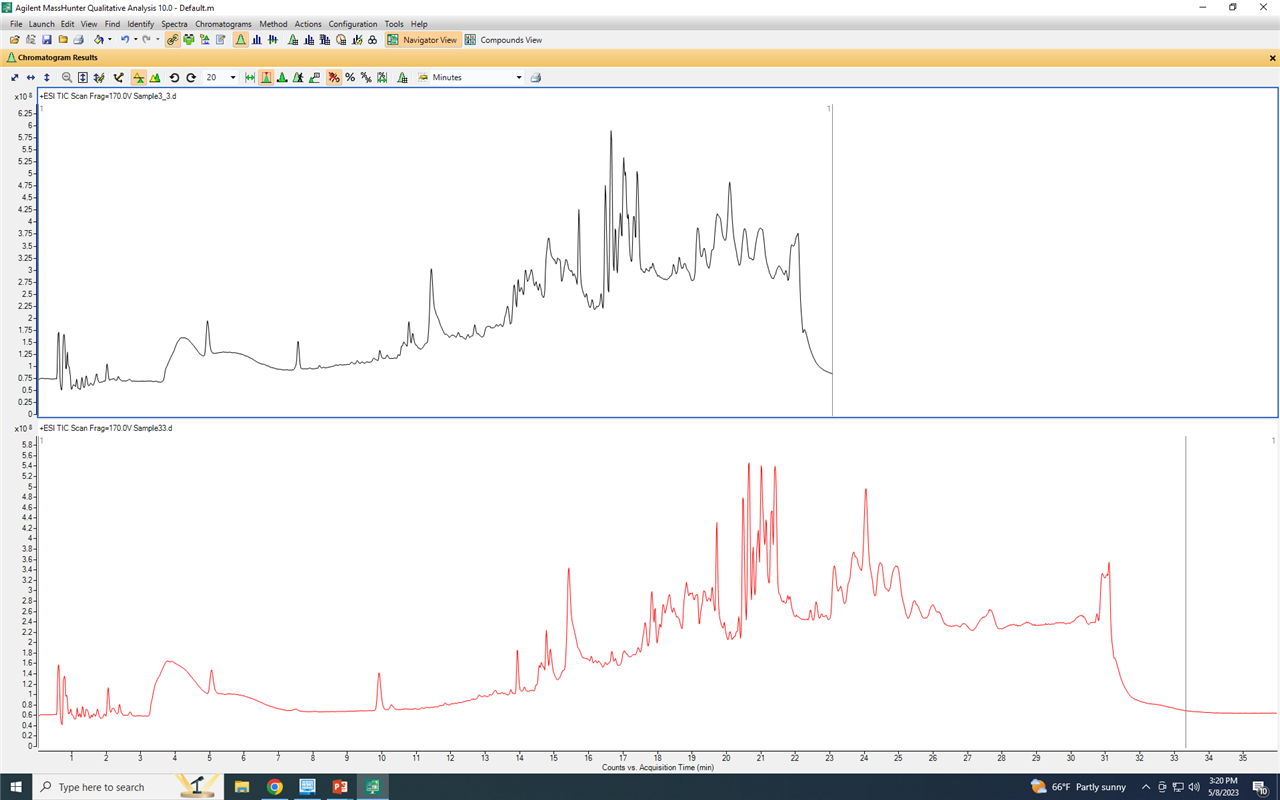
The BPC traces are as follows ( in both list and overlay modes)
It is supposed to be separated like the K13 sample (second from top) but somehow it starts to be broadened and not separeted in most of the samples.
The sample(s) are methanol extracted plasma (5 ul injection volume).
I initially suspected column overlading but dilution did not help.
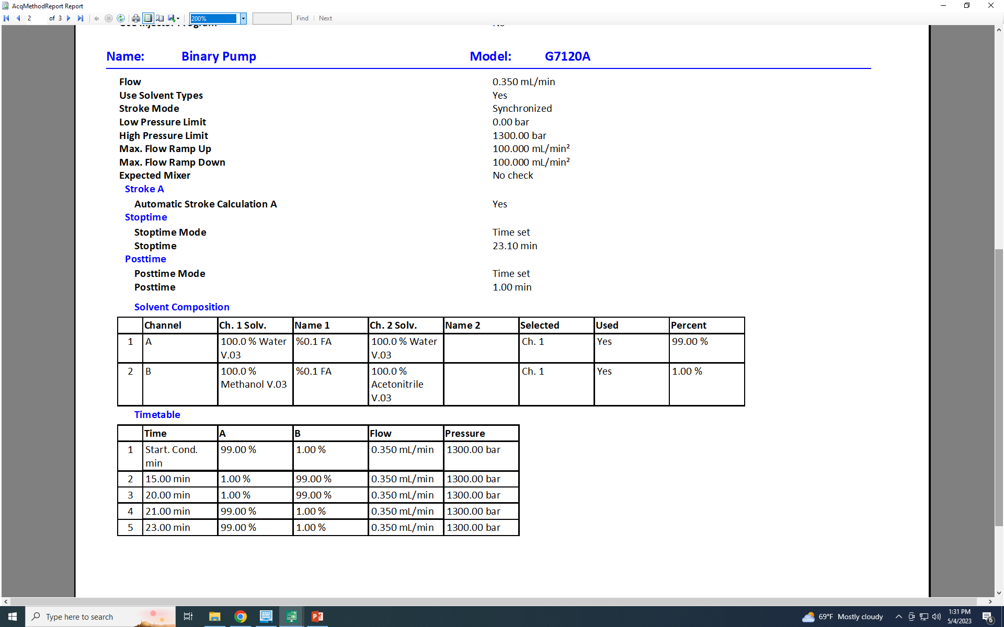
The second thought was the that the jump from A% to B% was steep and I have also slightly added a 4 mins isocratic step again not much improvement.
The only thing I have not tried is to increase the gradient time from 23 mins to sth like 40-50 mins. But this also does not make sense to me as the column is HSST3 (1.8 um, 50 mm, 2.1 mm)
Any possible solution or suggestions?
Thanks in advance,
Basri