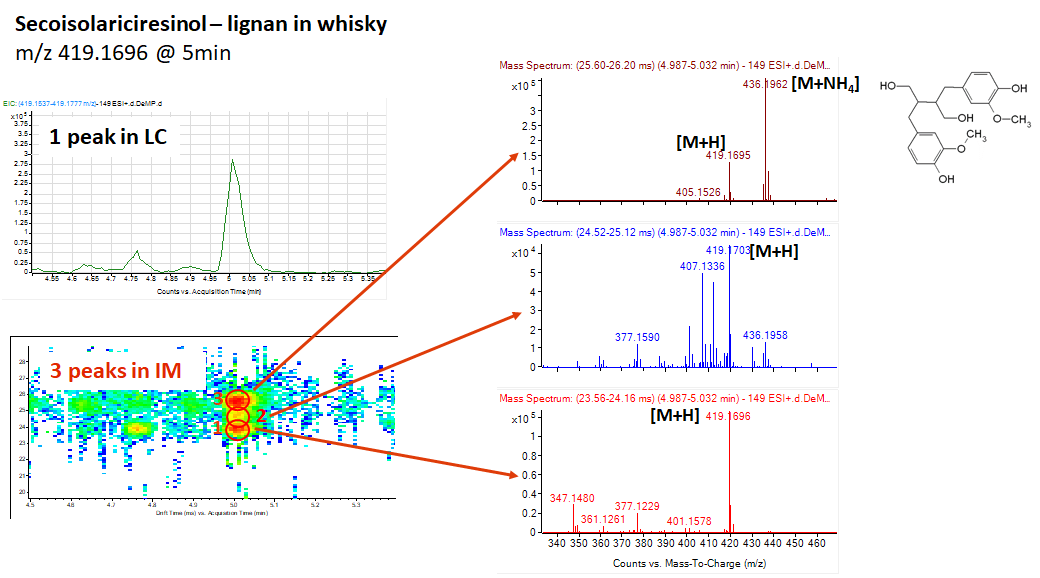
Recently we purchase an Ion mobility QTOF (IMMS) and run simple
application with small molecules that shows 3 peaks for our marker.
A question raised from our colleagues about the possibility that IMMS can separate
the same molecule that protonated on different spot of the molecule.
Does anyone can answer this question? Did IMMS separate between same
molecule with different protonated site (e.g. NH2+ vs. OH2+)?