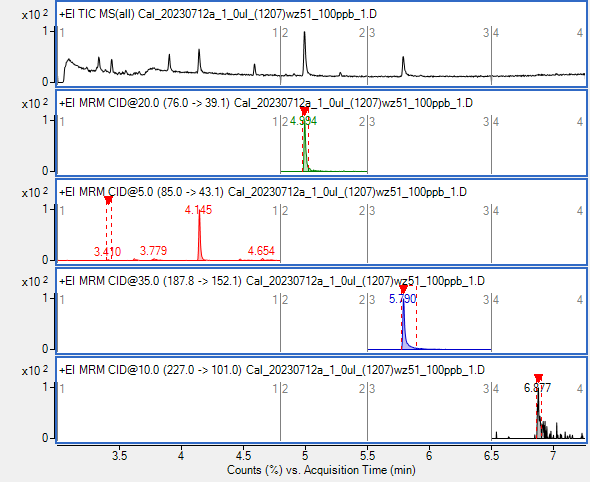
Can I use Agilent checkout sample 05970-60045 containing biphenyl, p-chlorodiphenyl, and methyl palmitate in isooctane (100ppb, 10ppm and 100ppm) for PQ- performance verification (just 100ppb and 10ppm only)? There is no certificate in the box for these compounds, methyl palmitate at 100ppb has low signal. What is your opinion , please?