Which Electron Ionization Source Part 1 has questions. Which Electron Ionization Source Part 2 has a few answers. Which Electron Ionization Source Part 3 has the particulars of each source.
This segment - Which Electron Ionization Ion Source Part 4 - ties it together.
Will the carrier gas be hydrogen? Running hydrogen does not eliminate problems caused by the gas supply, high sample throughput, system overloading, lack of sample preparation, poor methods, or lack of maintenance.
- Is the spectral fidelity for some very active compounds going to be compared to calculated spectra or library entries created using helium where a very high match quality is required? If that is the case, choose the HydroInert Ion Source.
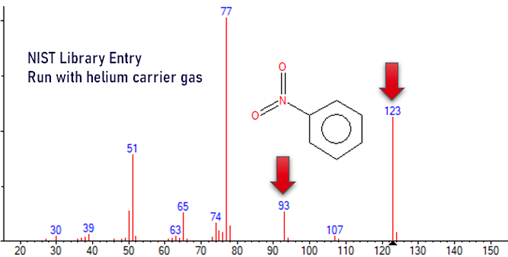
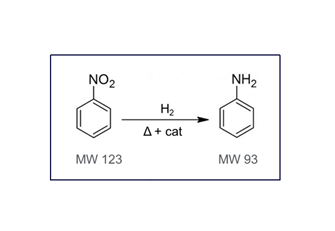
- With hydrogen in the ion source instead of helium, the spectra of some compounds can be different due to various different chemical interactions. One example is nitrobenzene, see pictures below.
- Nitro-compounds like nitrobenzene, fenitrothion, ethalfluralin
- Heavily chlorinated compounds like DDT, Endrin, heptachlor, BHC compounds, pentachlorophenol
- Pesticides like Deltamethrin, fipronil, permethrin, captan
- Fragrance/Flavors compounds like musk ketone, musk ambrette, linalool
- The spectra won’t match but the overall detector response will be the same.
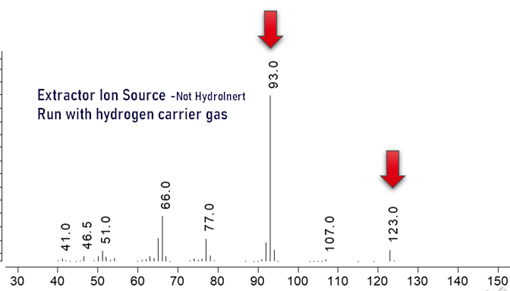
- If hydrogen is chosen for cost savings and availability, any ion source may be used, see below.
- For more information:
Do you need versatility in sample peak concentrations or amounts?
- HES: Are the peaks of interest parts per quadrillion (ppq) or parts per trillion (ppt) concentrations? If it is used properly and required to see the lowest levels possible the HES may be the right choice. If any sample peaks are higher concentrations the HES may result in an increased level of user maintenance and the extractor source may be the right choice.
- Extractor: The extractor ion source may be used with Etune to give higher ion transmission than the SS or Inert or used with Atune to be similar to the SS or Inert. The standard 3 mm extractor lens could be replaced with a 6 or 9 mm to raise the upper end of the dynamic range by giving away the bottom end.
3, 6, and 9 mm Drawout Plates for the SS/Inert Ion Source on the top
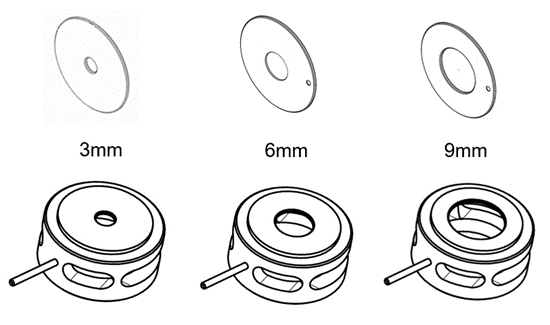
3, 6, and 9 mm Extractor Lenses for the Extractor Ion Source on the bottom

- SS/Inert: If your typical samples are parts per billion or higher, consider the Stainless Steel or Inert ion source. The standard 3 mm drawout lens could be replaced with a 6 or 9 mm to raise the upper end of the dynamic range by giving away the bottom end.
Is your application well defined, already run on many instruments?
- Find out what ion sources are typically used
- Application notes by instrument: 5977, 7000, 7010, 7250
- Search Agilent.com using the J&W GC Column Selection Guide for the column type, scroll down to Product Details then select Applications to see a list, and scroll further down that list for application notes using that column.
- Application-Specific GC/MS Solutions – databases, libraries, and analyzers.
- Talk to your salesperson
- Talk to a pre-sales application chemist or product specialist to talk about specifics and even schedule a demonstration
Are your samples complete unknowns? Do you know the compound types? The chemistry? The pH? The concentration range? Do you screen samples using a GC detector but need library search matching? How much sample preparation is involved? How much variability is acceptable? How much dynamic range is required? If your samples are in this category, it is important to investigate further. It is possible to destroy a GC/MS ion source and the quadrupole in a very few injections.
- Talk to your salesperson
- Talk to a pre-sales application chemist or product specialist to talk about specifics and even schedule a demonstration
If I was required to choose a system to purchase without knowing all of the necessary parameters about the samples, I would choose an Extractor Ion Source system along with 6 and 9 mm extractor lenses. After the installation checkout was completed, I would install the 9 mm extractor lens, increase the ion source to 275 °C, and run Atune understanding that the 502 relative abundance will be slightly lower than at 230 °C but willing to trade that off for long-term detector cleanliness. Then I would do a serial dilution of samples, start with the lowest possible concentrations and work up in concentration until peaks are seen in the low thousands of counts before continuing with application development. I would consider the sample dilution strategy before trying the 6 mm or standard 3 mm extractor and only then try Etune or higher EM Gain to be able to see the levels injected.
To maximize the instrument’s lifetime, reduce the amount of routine user maintenance, and use the least number of consumable parts, run a GC/MS with the smallest injected volume of the lowest possible concentrations of the cleanest samples.
All the currently available Electron Ionization Ion Sources: Stainless Steel, Inert, Extractor, HydroInert, and HES
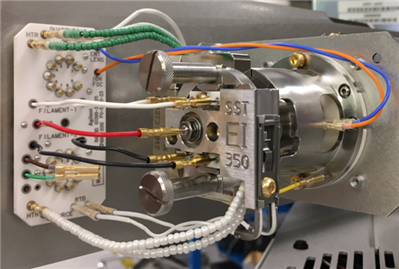
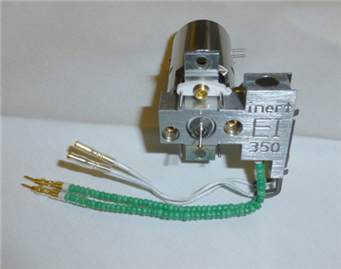
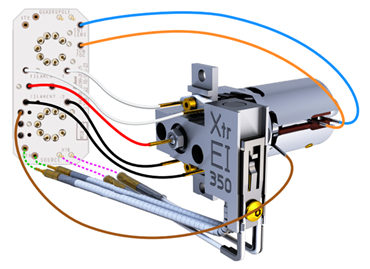

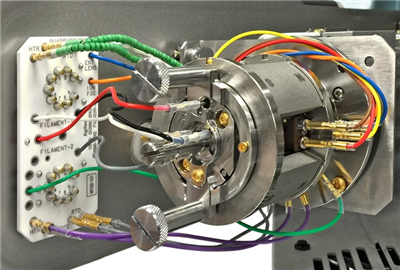
High Efficiency Ion Source 2.0 - new on the 7010D
DE11439015
.
.
.
.
.
