Hello
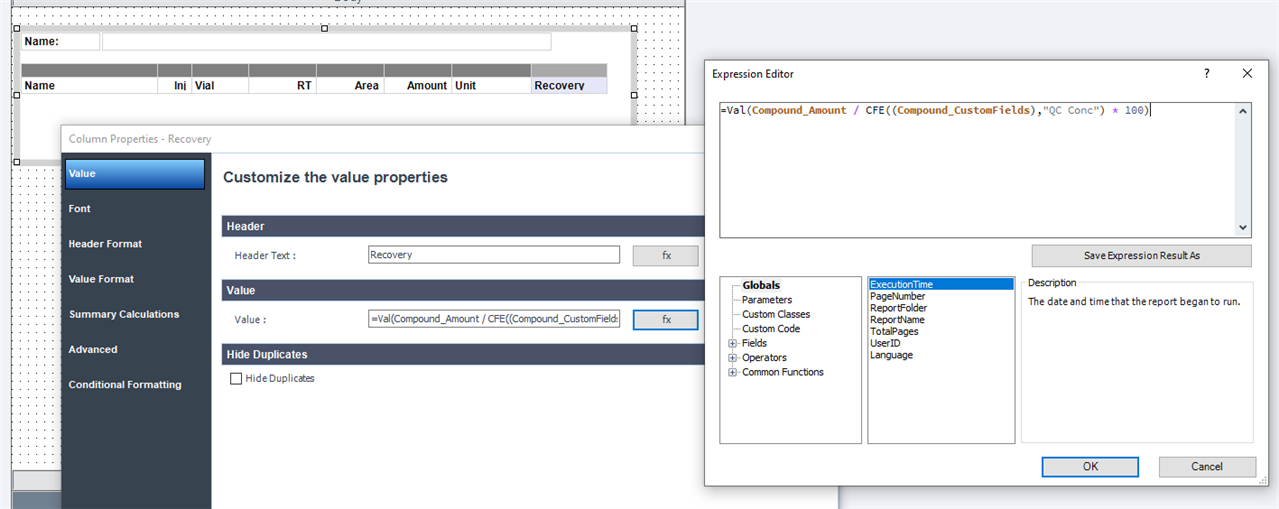
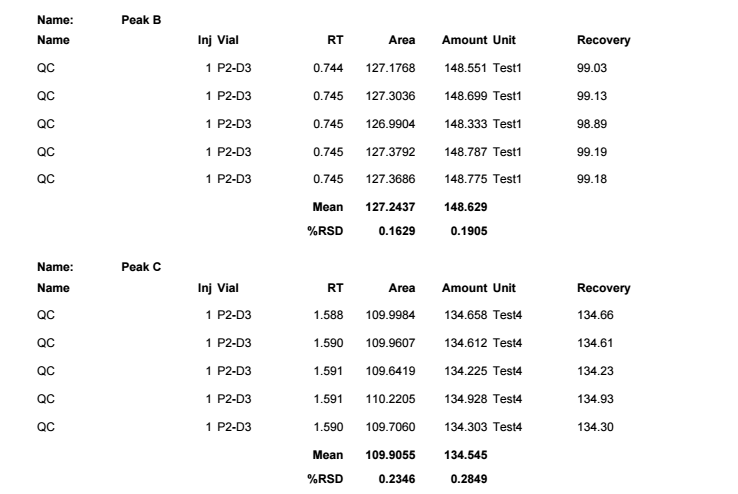
Is there any way that I can calculate automatically %diff between concentration for sample and calibration standard (for the same compounds)? At the moment I can display %diff for calibration standard but I want to do it for sample - I'd like to check how close real value (from sample) is to calibration standard concentration. It is quite useful to check if calibration curve still works.
Once I change sample type to SAMPLE, % diff is not calculated anymore. And if I change sample type to CAL it is updating calibration level which is affecting final result.
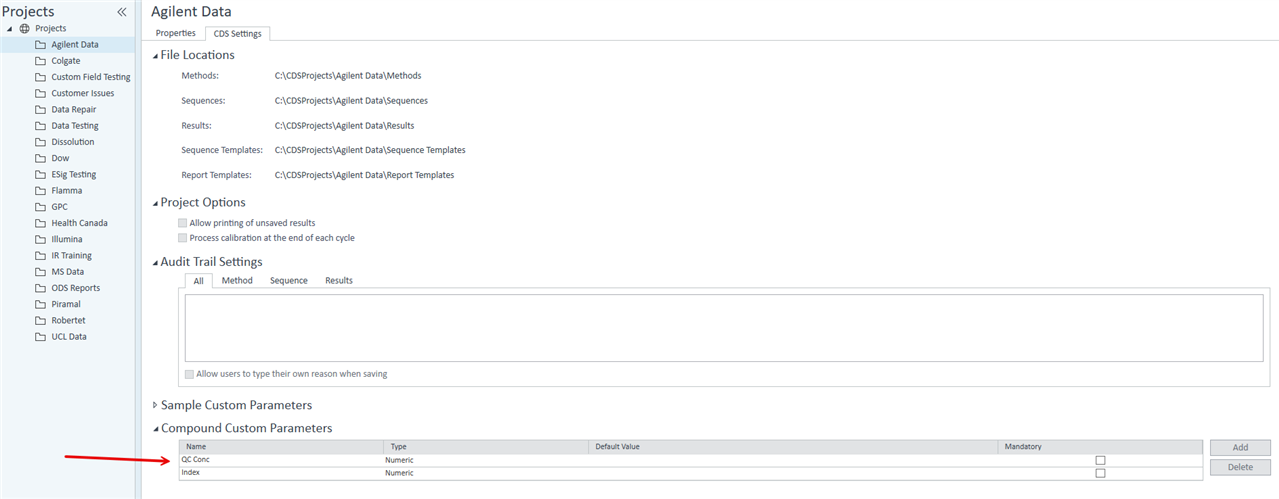
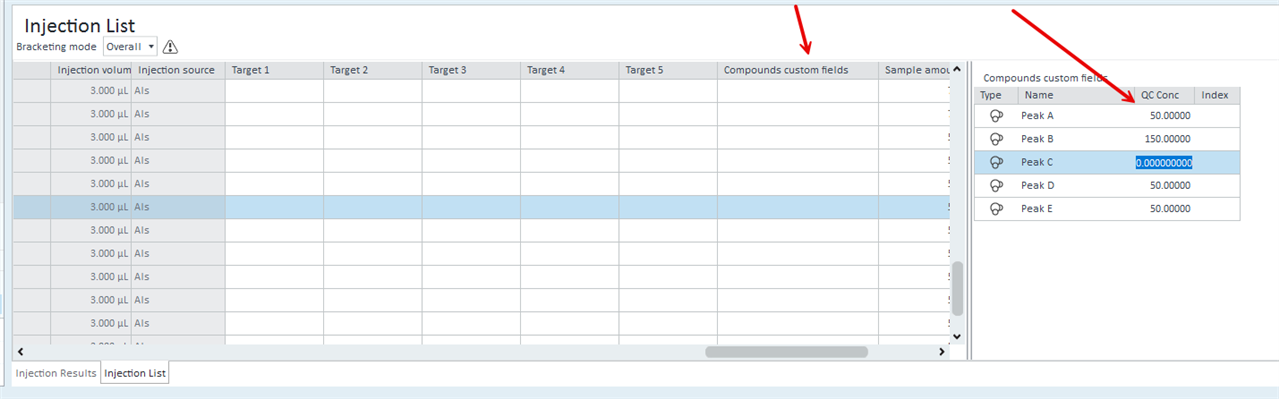
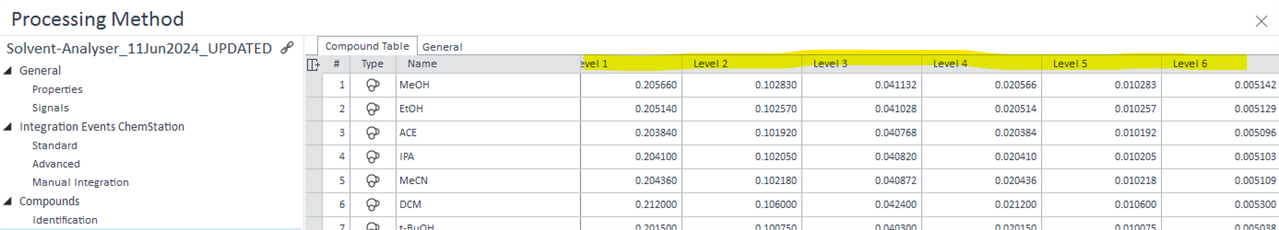
Alternatively: is there any way that I can display specific Cal Level concentrations in customized table? (see below)
Thanks
Regards
Tom