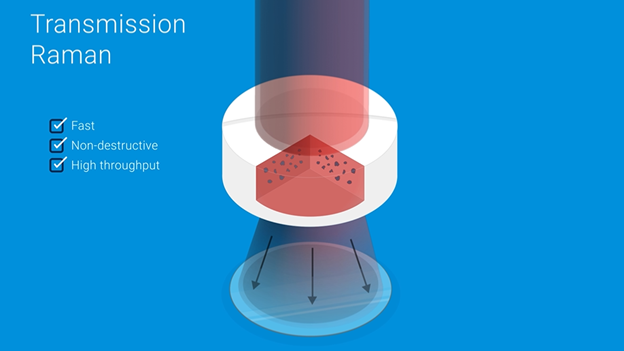
It’s all about transmission
Raman spectroscopy has already demonstrated itself to be a useful analytical method in laboratory and process settings, owing to straightforward optical setups, highly specific chemical signatures in spectra, and the ability to analyze solids, liquids, and even gases. Raman instruments in the market today typically measure light diffusely reflected (i.e., “back-scattered”) from the surface of a sample, and thus generally take the form of Raman microscopes or probes. However, here at Agilent we have another innovative ace up our sleeve: Transmission Raman Spectroscopy (TRS).
Obtaining spectra truly representative of the entire sample can only be done via transmission analysis. A laser is positioned on one side of the sample, and as the laser scatters through the sample—generating Raman events—all of the light is captured on the other side of the sample and processed for analysis. This measurement paradigm, used by our TRS100 Quantitative Pharmaceutical Analysis System, allows characteristics of the whole sample to be revealed, rather than merely on the surface with reflective Raman techniques.
Watch this video to see how the TRS100 acquires data from a sample.
Shine light through a tablet and tell me what and how much is inside
The Agilent TRS100 Quantitative Pharmaceutical Analysis System was specifically designed for routine content uniformity analysis of oral dosage forms in pharmaceutical environments. This instrument can identify and quantify the contents of nearly any pharmaceutical sample, including tablets, capsules, powders, creams, and even liquids in vials. This is all done in a fully automated fashion, with detection limits down to about 1% w/w (weight by weight) concentration for most APIs (active pharmaceutical ingredients). The advantages are quite clear: no sample preparation, non-destructive testing, a more representative and therefore accurate result, and higher sample throughput than competing technologies.
Sounds too good to be true? Well, the old adage “without labour nothing prospers” applies here, but the initial effort to get the TRS100 into daily use easily pays for itself after very little time. Concentration calibration models are needed for the TRS100 to quantify production samples, and these models require standards with varying concentrations of API to build a concentration calibration curve (i.e., a mathematical relationship between signal strength and API concentration). These standards usually must be created—however most production facilities are more than aptly equipped to manufacture these with ease. Once this initial step has been completed, the TRS100 simply sails along performing content uniformity on a tray of a hundred samples or so at a time with minimal input from the user, and the savings become readily apparent.

TRS and HPLC - Agilent has got it all covered
Some of you enterprising readers might be thinking “This sounds great! Can TRS100 entirely replace HPLC?” For the highest confidence in results, the capabilities of the two technologies provide a fantastic synergy. For example, using HPLC to validate concentration models is a good practise for quantitative analysis. Some samples will have a very low concentration, or no Raman signal, and HPLC is well established for these applications.
The TRS100 is ideal for high volume sample analysis, where speed and routine quantitation are key. The overall efficiency increase of these techniques operating in parallel can be immense—time savings, cost savings, and reduced solvent consumption. Lastly, transmission Raman spectroscopy has recently been approved as a content uniformity technique by the FDA. It seems there’s no better time to get the laser firing and see if the TRS100 will benefit your tablet formulation or manufacturing processes!
Further reading
- Technology Overview: Transmission Raman Spectroscopy
- White paper: Implementing Transmission Raman Spectroscopy for Fast Content Uniformity Testing: From Feasibility Evaluation to a Validated Release Method
- Application note: Quantitative Determination of Salt-Form Impurities of Warfarin at the Lowest Commercial Dose Strength using Transmission Raman Spectroscopy
- Application note: Evaluating Repeatability and Sensitivity of Transmission Raman Spectroscopy
- Application note: Quantification and Identity Testing of Soft Gel Capsules using Transmission Raman Spectroscopy
- Application note: Quantification of Crystallinity Using Transmission Raman Spectroscopy
Molecular spectroscopy webinar series
Get the latest product information and lots of helpful tips and tricks from our frequent molecular spectroscopy webinars. These live and interactive sessions take place every week, with all previous sessions available to watch on-demand.
Got a question? Leave a note on the comments below or send your query directly to our expert spectroscopy team.