In this podcast, Dr. Melanie Zerulla-Wernitz, Head of the Analytical Science Laboratory at Vetter Pharma, discusses the tools and techniques her team uses to optimize analytics of oligonucleotide-based pharmaceuticals. Dr. Mathieu Rault of Agilent Technologies also highlights how to execute temperature-controlled measurements of oligonucleotides and outlines the implementation of spectroscopy tools for fast, accurate ID testing.
Hear about Vetter Pharma’s main activities, including work done around oligonucleotides and the challenges associated with them. Dr. Zerulla-Wernitz also shares how the Cary 3500 UV-Vis has helped Vetter Pharma to overcome these challenges.
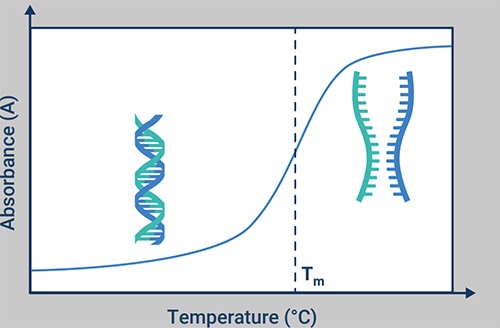
Dr. Rault goes on to discuss the factors to be considered when selecting tools and techniques for accurate oligonucleotides ID confirmation. He also expands on the importance of temperature control during oligonucleotide melting temperature analysis.
Listen to the podcast or learn more about how to optimize oligonucleotide melting temperature analysis from the following resources:
- Video: Run Your UV-Vis Thermal Melt Assay in Less Than 10 Minutes
- White paper: Best Practice for Nucleic Acid Thermal Stability Measurements Using the Cary 3500 UV-Vis Spectrophotometer
- Application note: Fast Determination of Thermal Melt Temperature of Double-Stranded Nucleic Acids by UV-Vis Spectroscopy
Cary 3500 UV-Vis and Cary UV Workstation
Complementing the Cary 3500 UV-Vis, the Cary UV Workstation software can be configured with OpenLab to help organizations meet their compliance goals. The OpenLab software suite includes optional secure database storage for electronic records, complete administration for users and access privileges, software licensing, and security settings—e-signature workflow and enhanced audit trail review capabilities.
The Cary 3500 UV-Vis spectrophotometer series uses powerful, advanced xenon flash lamp technology. This lamp helps to remove any warmup burden before using the instrument. The xenon flash lamp comes with 10-year replacement warranty (for Cary 3500 instruments purchased from Agilent or participating partners) and drastically reduces the frequency and cost of lamp replacement.

