Deaeration, or degassing, is the controlled process of removing excess dissolved gases, primarily air, from dissolution media. When performing dissolution testing, this can be one of the biggest headaches in the lab; and, if not done properly, is one of the most common causes of dissolution failures. This post combines expert advice from kenboda (Agilent) and Bryan Crist (DissoAssist) to give you an overview about why deaeration is important and how to go about this process most effectively.
Background and Importance of Deaeration
So, why do dissolved gasses cause such an issue in dissolution? Dissolution is essentially a sample preparation device, but one that occurs under precise and controlled conditions. In addition to all the physical parameters of the instrument, a maintaining a reproducible mixing environment and the hydrodynamics within is also critical. Dissolved gasses can lead to significant variability in dissolution, or skew results high or low, so it’s important to minimize this impact.
How can dissolved gasses in the vessel lead to different results? These gasses – specifically air bubbles – can act on the dissolution environment or your dosage form in different ways depending on the USP apparatus type. This can lead to a variety of issues:
- USP Apparatus 1 (Baskets) – bubbles can form on the basket mesh leading to insufficient or inconsistent product interaction with the dissolution media, leading to lower results. Bubbles can also form on the dosage form itself and either block dissolution or act as an additional force to erode your product. Effervescent tablets are an excellent way to have a rapid dissolution after all.
- USP Apparatus 2 (Paddles) – in disintegrating dosage forms or beaded formulations, the air bubbles can agglomerate particles or pull them to the surface of the media and slow the dissolution. Air bubbles can form on the vessels, paddle blade, etc. and alter the hydrodynamics in the vessel. Bubbles covering a paddle will “inflate” its dimensions, increase the mixing, and consequentially the dissolution rate.
- USP Apparatus 5 (Paddle over Disk) and 6 (Rotating Cylinder) – Similar to baskets, bubbles forming on the surface of a transdermal product reduce the amount of exposed surface area and can slow the rate of dissolution, causing lower results.

How to Deaerate/Degas Media
Deaeration can be done in several ways in the lab. The primary method to remove dissolved gasses is the method described in USP <711> Dissolution. This procedure, as well as alternate approaches, may also be used.
The USP method – After preparing the media as required, heat the media to 41 – 45°C, pulling it through a filter with a vacuum, and then keeping it under vacuum for an additional 5 minutes. This approach can be time consuming if using a single layer filter, so a multi-layer filter is often used to accelerate the process. The setup commonly requires the use of a heating/stir plate, vacuum source with moisture trap, two preparation bottles built to withstand low pressure vacuum, and appropriate filter. This technique should be performed within a safe area, such as a laboratory fume hood.
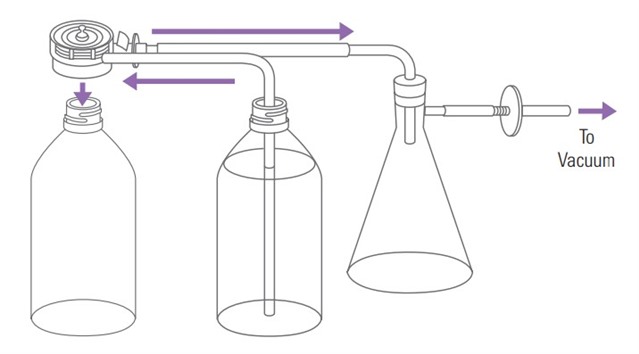
Alternate deaeration methods that a lab wants to use should be compared to the USP degassing procedure and validated to show that similar results are achievable. This comparison can be done through product testing with the USP degassing approach and alternate degassing approach and demonstrating equivalence using an f2 calculation (similarity factor). The comparison can also be done to show that the alternate degassing approach can get < 6 ppm dissolved gasses using a total dissolved gasses meter.
Examples (and comments) of alternate degassing methods include:
- Helium (He) sparging – Helium has become variable in price and there have been issues with supply shortages over the last several years. Secondly, helium sparging is often not properly defined in methods and failures may occur due to insufficient time or incorrect flow. Be sure to specify these necessary conditions – for each product – in the dissolution method to ensure consistent results.
- Superheating – this technique involves heating to a higher level without use of a vacuum, and could work for certain products, but will require a longer cool down time before the media comes back down to an appropriate temperature for the dissolution test.
- Media degassing stations – these stationary or portable units may be used successfully but should be validated carefully to ensure adequate deaeration for each product.
- Nitrogen (N) sparging – this should be considered an unacceptable method of deaeration since it super-aerates the media.
- Sonication – could be attempted but is effective for aqueous only solutions.
PRO TIP(s)… stay on task!
Degassed dissolution media is not in a state of equilibrium. Once your media has been deaerated, you should move efficiently to ensure that it stays that way.
- Pour the media carefully so that you don’t re-introduce dissolved gasses back into the media.
- Weighing dissolution media directly into vessels can reduce the number of times you pour the media and offers great volume accuracy.
- Use your media as soon as possible after it has been de-aerated. Media with surfactants can re-aerate fully in 15 minutes, media without surfactant may re-aerate fully in 30-60 minutes.
Once media is deaerated, this isn’t time to go to lunch, check your e-mails, or attend a meeting! If performing the USP method of deaeration, the media will typically be about 39 – 41°C when poured into the vessels and you should be at 37 C +/- 0.5°C within about 5 – 10 minutes. This time can be used to prepare your sampling cannulas, filters, etc. or label your sample tubes/vials and you’ll be ready to drop your dosage in no time.
When to Deaerate/Degas Media
USP General Chapter <711> states that “dissolved gases can cause bubbles to form, which may change the results of the test. If dissolved gasses influence the dissolution results, dissolved gases should be removed prior to testing.” Given all the different ways that air bubbles can potentially impact the dissolution test, it would seem like removing these dissolved gasses through de-aeration would be mandatory, but this isn’t necessarily true. Some methods and products are not sensitive to dissolved gasses. If you can show through a validation that this is the case, then you can choose to not include media deaeration for that method. The validation process should be performed for each product/method and typically includes an f2 comparison of n=12 degassed vs. n=12 non-degassed media. The USP degassing method is most often used for this type of validation.
Some additional helpful hints learned through the years regarding media deaeration:
- USP Apparatus 3 (Reciprocating Cylinder) tests do not require media deaeration due to the rigorous mixing environment.
- Media containing surfactants are also less likely to require de-aeration since this media will reaerate more quickly than media without surfactants. In some cases, this media could fully reaerate in as little as 15 minutes.
- Extended-release products that are non-disintegrating also tend to be less impacted by dissolved gasses and worth investigating to see if dissolved gasses need to be removed.
Summary
Proper media deaeration is critical for many products to have a proper and reproducible dissolution test. The USP procedure of heating to 41 – 45 °C under vacuum is the primary approach, but there are other acceptable procedures when validated properly. Not all products require deaeration, and you can easily validate this through comparative testing. If your method does require deaeration, this is a media which is not under equilibrium and proper care must be taken to it remains deaerated when the dissolution test is started.
For further reading, check out this article – “Media Deaeration: What’s All the Hot Air About?”
If you have comments, questions, suggestions, corrections, etc. let us know in the comments. Additional support is always available at dissolution.hotline@agilent.com. Thank you for reading!
