(originally posted by Ken Boda, Dissolution Product Specialist on LinkedIn)
One question I frequently get is about Q in the USP. What is Q? How to I determine Q? How do I interpret the acceptance tables?
USP defines Q as the quantity or the "amount of dissolved Active Pharmaceutical Ingredient (API) specified in an individual monograph, expressed as a percentage of the labeled content of the dosage unit...". When we look at a Q value, we are looking at what percent has dissolved at that time for that product. Using a percent value as a standard allows for easier standardization across all the monographs in the USP and also helps to more easily apply limits based on statistics. Having the tables defined in USP <711> and <724> instead of the individual monographs also helps to greatly reduce the size of each monograph as well!
The Acceptance Tables are broken down into several categories, depending on what type of dosage form is being tested. You will find tables for Immediate-Release Dosage Forms, Immediate-Release Dosage Forms Pooled Sample, Extended-Release Dosage Forms, and Delayed-Release Dosage Forms. Acceptance criteria in the USP are based upon 12 samples, otherwise seen as n=12. In the acceptance tables, this is Stage 2. In stage 2, you will see that your average release is equal to Q. Stage 1 is essentially a shortcut based on statistics where you can pass the test with only 6 samples, or n=6, using a tighter set of acceptance criteria. If n=12 doesn't pass, then you move to stage 3, where you have a total of 24 samples or n=24. In Stage 3, you have a larger sample size, so the statistics allow for a wider range of acceptance.
The Immediate-Release acceptance table is as follows:
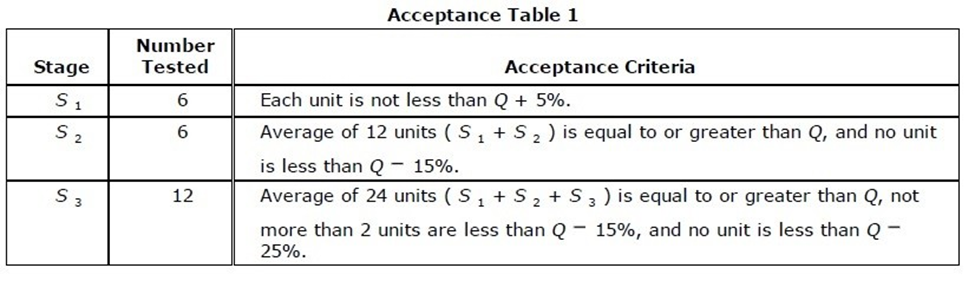
Let's assume that Q = 85% dissolved. Using this, then our acceptance criteria for this table would be:
- S1 - 6 units tested. Each unit is not less than 90% (Q+5%)
- S2 - 6 additional units tested. Average of 12 units (Stage 1 plus Stage 2) is equal or greater than 85% (Q), and no unit is less than 70% (Q-15%).
- S3 - 12 additional units tested. Average of 24 units (Stages 1 + 2 + 3) is equal to or greater than 85% (Q), not more than 2 units are less than 70% (Q-15%), and no unit is less than 60% (Q-25%).
Some things to note here. You see that the specifications loosen as the sample size increases - this is due to increased confidence in statistics. You are also allowed limited outliers which can be well below Q in Stages 2 and 3.
For Immediate Release with Pooled Sampling, the table looks quite a bit different. Here, you are taking a Pooled Sample - basically 1 sample which is a mixture of the n=6 to n=12 of that stage. Since you're only testing 1 sample per stage, you are testing fewer samples and the statistical requirements therefore are much tighter.
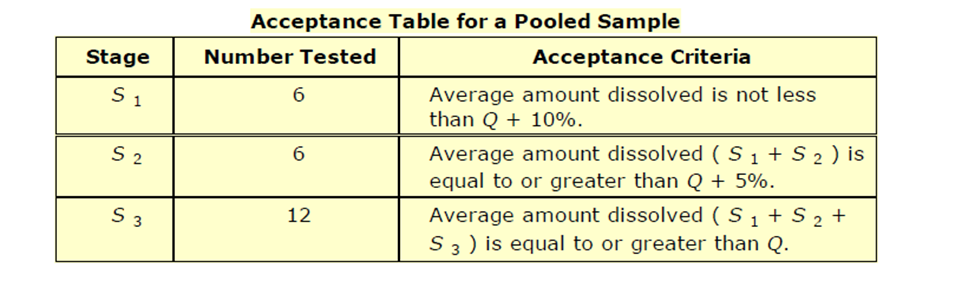
Using the same Q = 85%, your acceptance criteria would be:
- S1 - 95%
- S2 - 90%
- S3 - 85%
For Extended Release Products, you are now dealing with acceptance ranges. You must meet the table for each specification point in the dissolution monograph. It isn't uncommon to have 3 individual specifications, each which must be met in the following table:
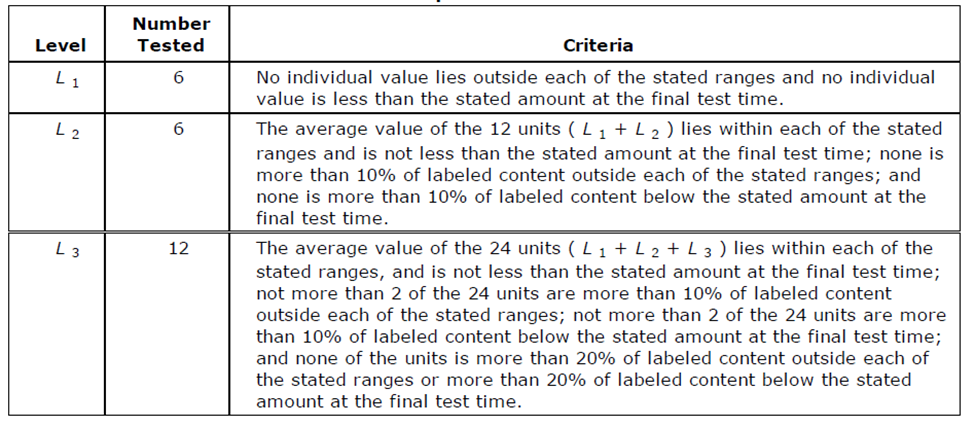
Let's assume for this table, that we are choosing a later timepoint and our acceptance criteria range is 70-80%. Our acceptable limits in the table would then be:
- L1 - 70-80%
- L2 - Average of 70-80%. No individual dose is outside of 60-90%.
- L3 - Average of 70-80%. Only 2 units can be outside of 60-90%. No units can be outside of of 50-100%.
If we have 3 timepoints, you would need to confirm that each of these 3 timepoints meets this acceptance table.
Delayed-Release Products have a separate acceptance table to allow for an acidic challenge and buffer stage. In the acidic challenge, you ideally want to see nothing dissolved indicating that the dose is fully protected from the stomach acid. Please note that this table is not compendial in the Japanese Pharmacopeia.
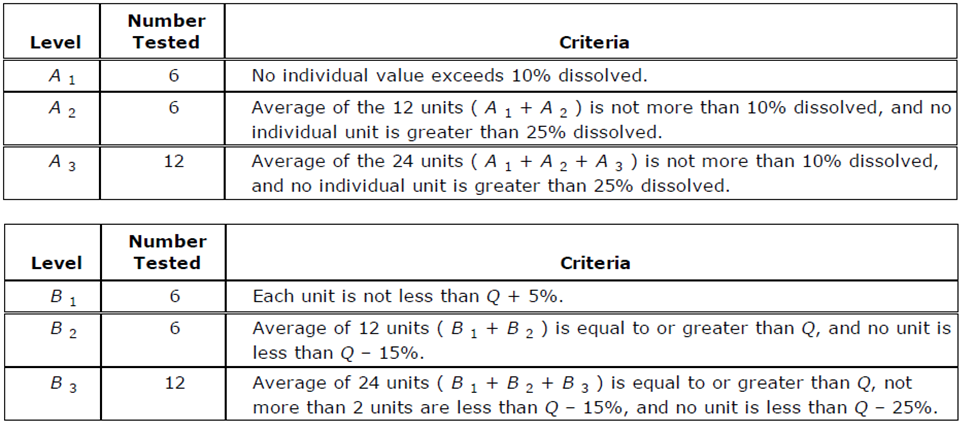
In the delayed-release tables, you have one for the Acid Phase (A) and another for the Buffer Phase (B). The Acid Phase is pretty self-explanatory. In the Buffer Stage, you see something very similar to the Immediate-Release table above. If we assume Q = 85% again for the Buffer Stage, our passing values would be:
- B1 - Each unit is not less than 90%
- B2 - Average of 12 units is equal than or greater to 85%, and no unit is less than 70%.
- B3 - Average of 24 units is equal or greater to 85%, not more than 2 units are less than 70%, and no unit is less than 60%.
I hope this walkthrough of Q in USP <711> is helpful. For further clarification, feel free to contact Agilent's dissolution experts at dissolution.hotline@agilent.com.
DE46758677
