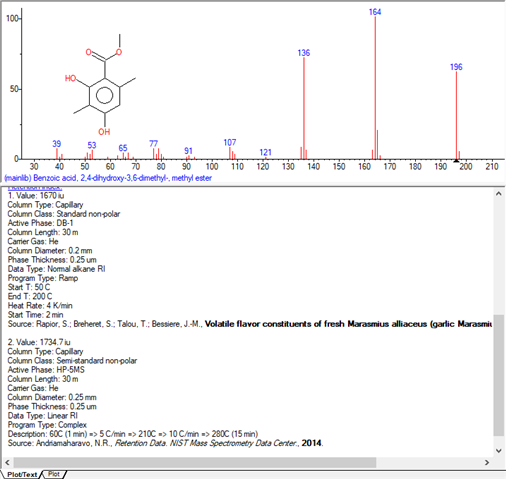
I am working with a DB-WAX column from Agilent, trying to identify components of a fragrance. In my analysis, the samples are vaporized at 250°C and the oven programing is from 50 to 250 °C. A chemical called methyl beta-orcinol carboxylate (Oakmoss, Evernyl, Veramoss) does not get detected, when injected in pure form or as a part of some fragrances. In others, a small peak appears towards the end of the temp programing (@250°C). Any idea what causes this? Does it have to do with chemical properties of the sample or column chemistry? Do organic acids behave differently in DB-WAX?