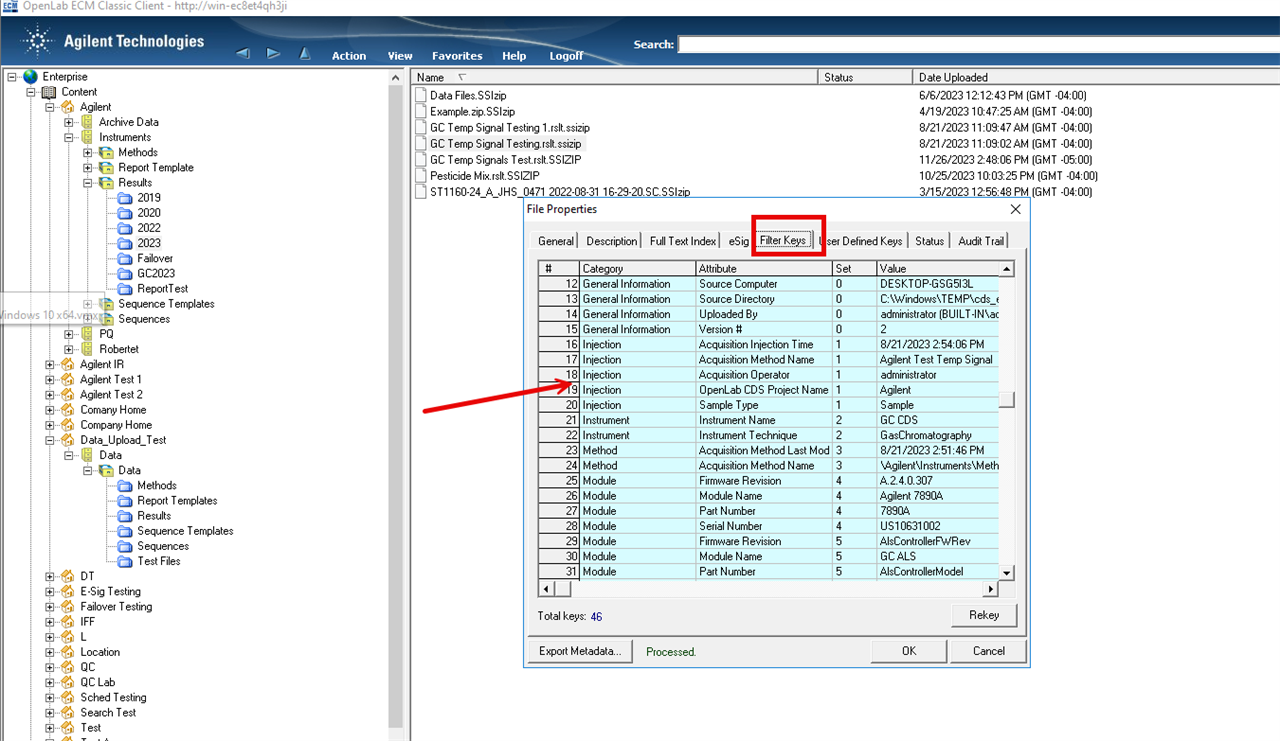
I'm currently writing an SOP regarding OpenLab administration. Specifically, I'm having trouble determining the best method of identifying orphan data as it relates to review of chromatography data in the GMP laboratory. Orphan data is unusual in that it could indicate that an analyst is intentionally placing data in another location not obvious to the reviewer (e.g. test injections for problematic method run prior to full sequence). There are two ways that I know of to perform a search.
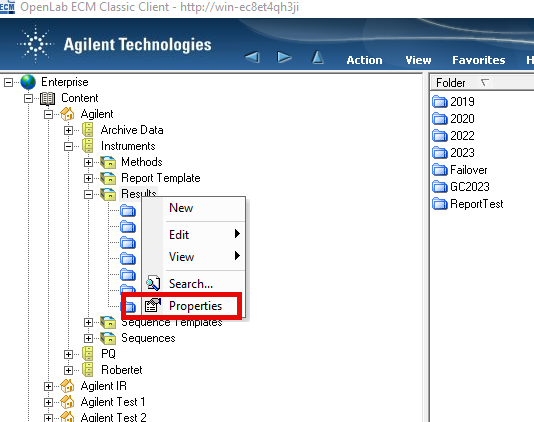
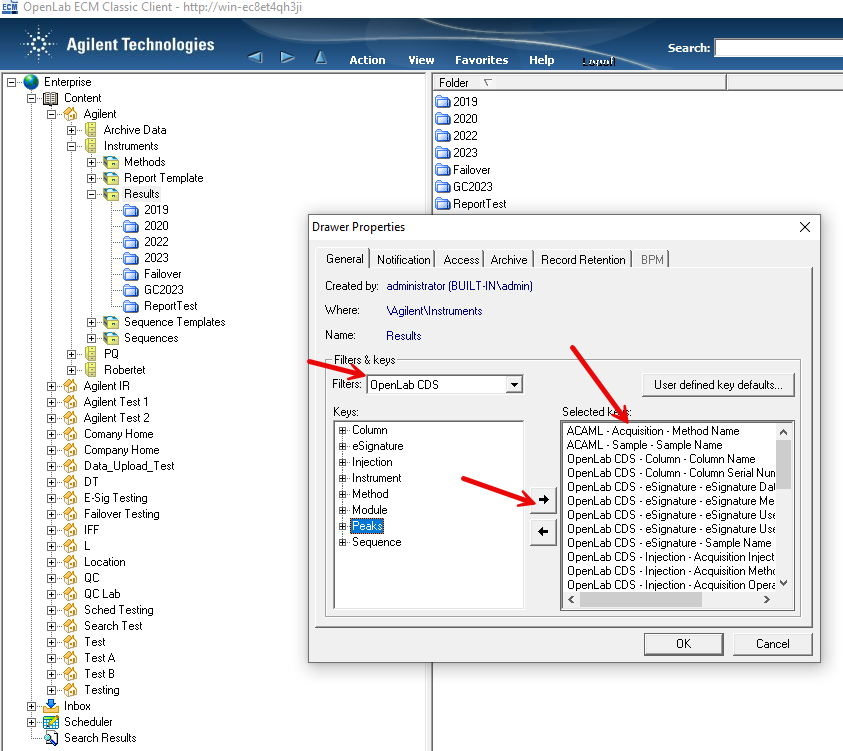
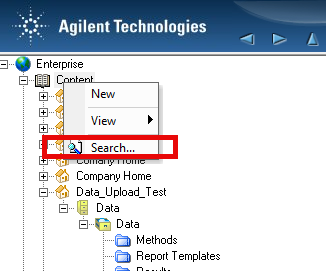
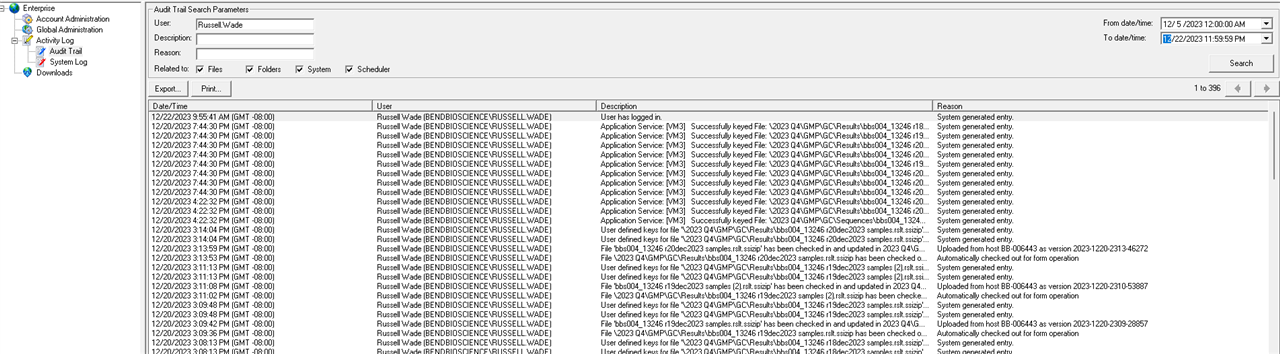
1. Via ECM classic client and performing a filtered audit trail search using user name and/or dates. The populated information doesn't seem particularly useful to a reviewer/auditor IMHO.
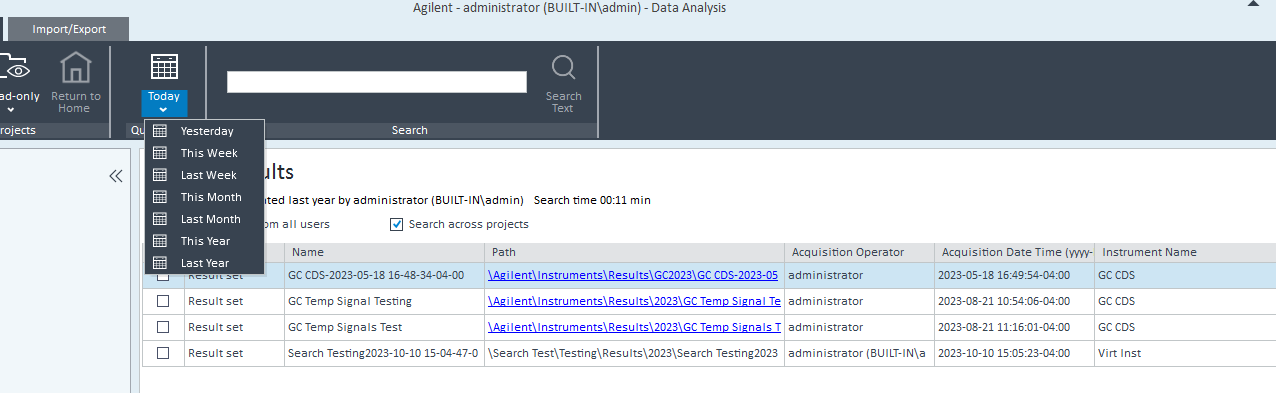
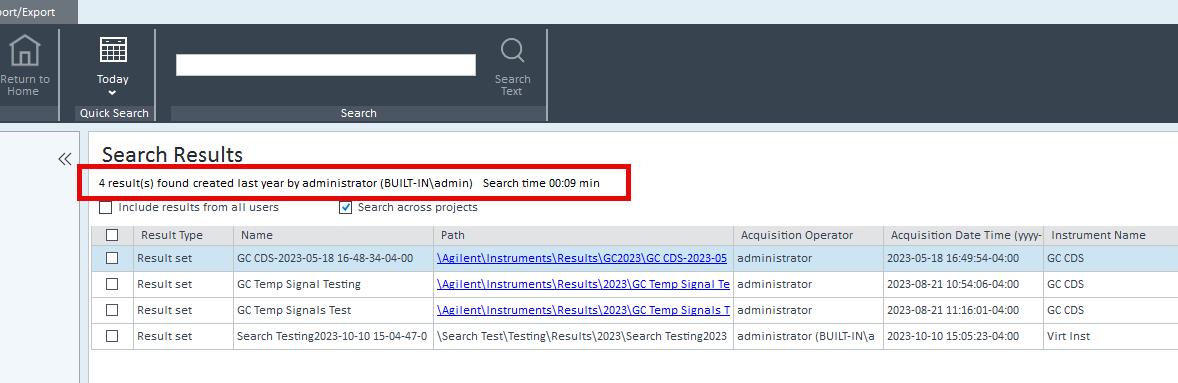
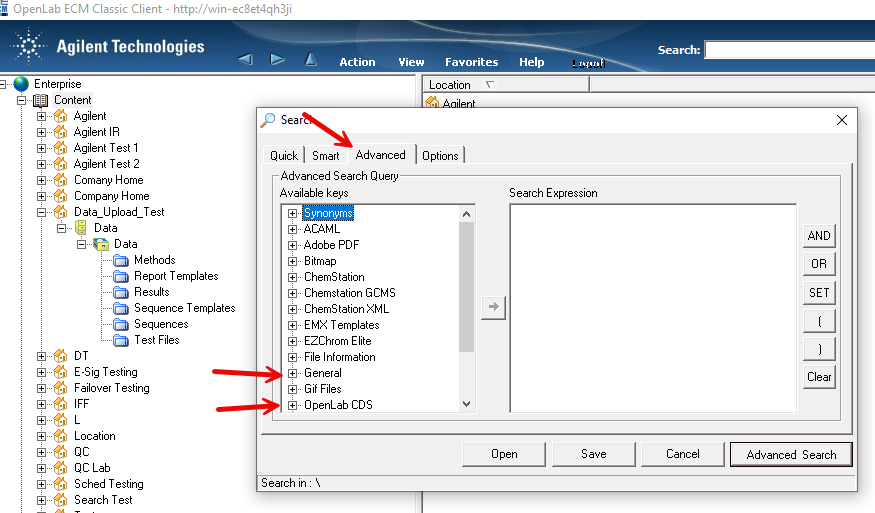
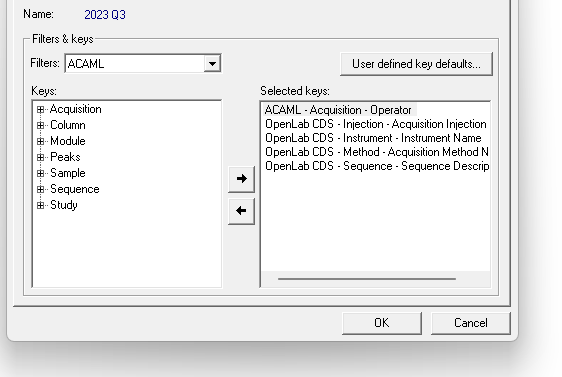
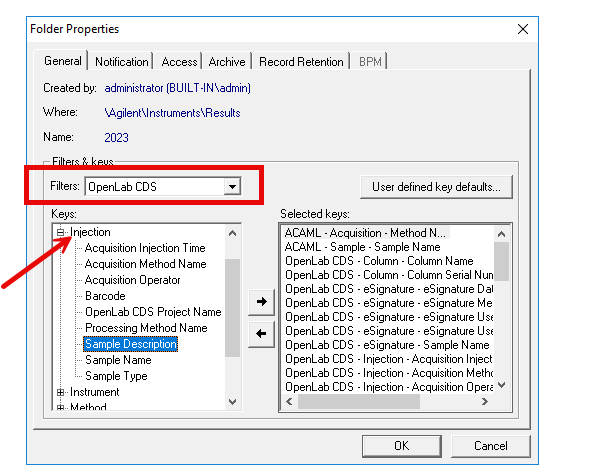
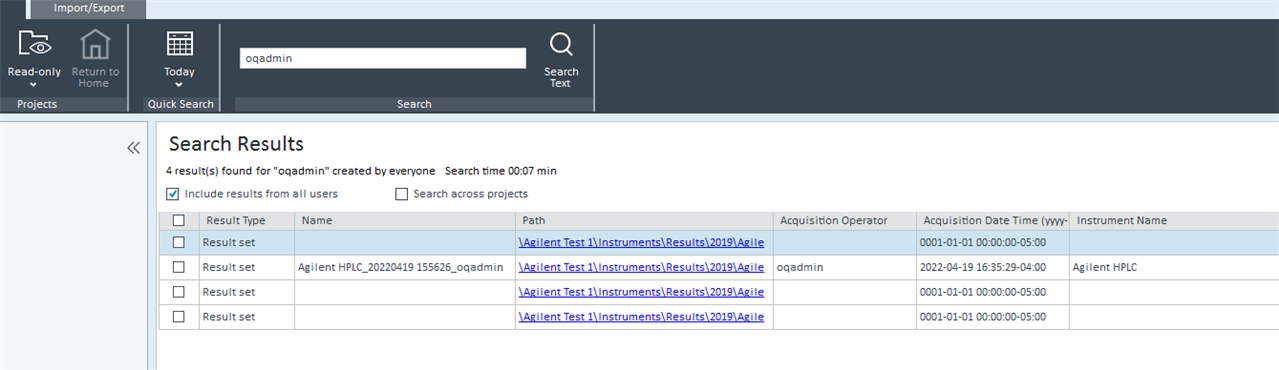
2. Via Control Panel/Data Analysis and Search Results. Include results from all users and Search across projects boxes are both checked. This seems like the best route but it seems that it isn't possible to customize the search by date. Although you can use the quick search option. In my case, if I use the quick search option, no data is found.
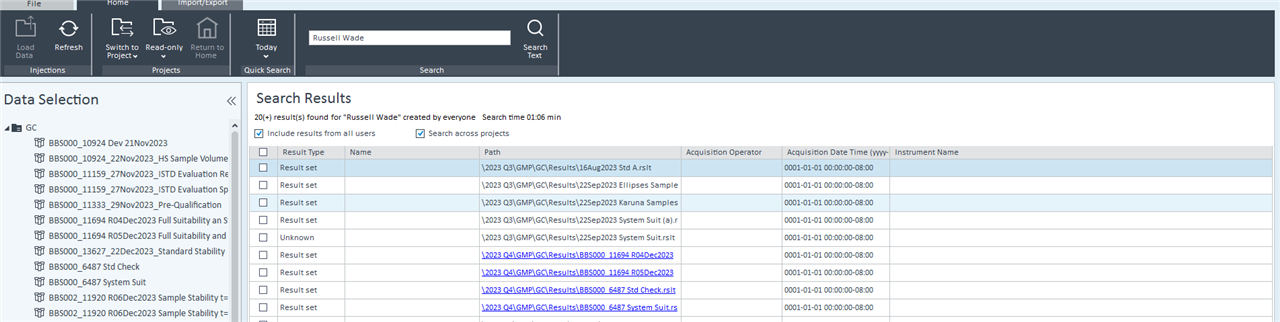
Any help or insight that you can provide would be appreciated!