Hello,
I am running an Agilent 1100 series with a quat pump. The column is Agilent Poroshell 120 EC-C18 3.0x50mmx2.7um. Column compartment is at 50C. UV signal is 228nm. The mobile phases are 0.1% formic acid in water and 0.05% formic acid in methanol with a flow rate of 1.0mL/min. The gradient looks like this:
Time Mobile Phase A Mobile Phase B
0 40 60
1.0 40 60
7.0 23 77
8.2 5 95
9.5 5 95
10 40 60
13 40 60
I have been getting a consistent UV baseline during injections during the few weeks I've been running this system. Then I had to replace MPB and started getting a different baseline profile. I remade the mobile phase using newly opened methanol and cleaned all glassware used prior, with the same result. I tried making new MPA as well without any success. Nothing on the system changed in between these events so I assume it is related to the mobile phase but I cannot get the baseline to look how it did before. Here is an example below where the blue trace was how it looked before and the green trace is now it is looking now. The baseline is declining as the organic phase ramps in the gradient.
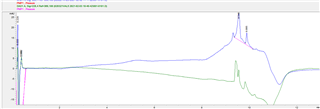
Any thoughts on what could be attributing to this?
Thank you!
